|
I Herr1, C Posovsky1, T Bohler2 & K-M Debatin2
1Deutsches Krebsforschungszentrum, Pediatric Oncology – D0800, Im Neuenheimer Feld 280, 69120 Heidelberg, Germany
2Universitäts Kinderklinik, Prittwitzstraße 43, 89075 Ulm, Germany
|
Dear Editor, Deregulated CD95-ligand (CD95-L/Fas-L/APO1-L) protein expression is suggested to be a major pathological mechanism underlying several disorders. Many of these CD95-L alterations were described based on the use of mAb 33 from Transduction Laboratories (Lexington, KY, USA). Since the specifity of this antibody is unclear,1,2,3 we have established 293 cells stably transfected with vector encoding the full length human CD95-L cDNA. Overexpression of CD95-L mRNA was ensured by RT – PCR4 (Figure 1A). Soluble CD95-L in supernatant of these cells induced death in target cells which was prevented by the CD95-L neutralizing mAb NOK-1 (Pharmingen, San Diego, CA, USA) or by blocking F(ab)2 anti-CD95 antibody fragments5 (Figure 1B). Using Western blotting,4 mAb 33 and other commercially available CD95-L monoclonal mouse IgG1 antibodies such as G247-4 (Pharmingen, San Diego, CA, USA) and NOK-1 yielded a band at the appropriate size of 37 kD which was increased in CD95-L-transfected cells (Figure 1C). This suggests that CD95-L protein was specifically recognized by G247-4, NOK-1 and mAb 33. However, while mAb 33 produced a single band, several bands were visible using G247-4 or NOK-1 which might be due to glycosylated forms6 or a high background binding. Another difference between these antibodies is their affinity to CD95-L protein which was highest for mAb 33 (10  g protein loaded, 10 s exposure time), followed by NOK-1 (10 g protein loaded, 10 s exposure time), followed by NOK-1 (10  g protein loaded, 5 min exposure time) and lowest for G247-4 (50 g protein loaded, 5 min exposure time) and lowest for G247-4 (50  g protein loaded, 45 min exposure time). Therefore, mAb 33 is highly sensitive in a Western blot assay and loading too much protein extract may saturate the ECL-reaction masking differences between low or high level expression of CD95-L.1,2 To further characterize G247-4, NOK-1 and mAb 33, the binding properties to recombinant CD95-L (rCD95-L) from Alexis (Grünberg, Germany) were evaluated. rCD95-L corresponds to the extracellular domain of human CD95-L and is fused at the N-terminus to a linker peptide and a tag. After SDS – PAGE and Western blotting, a specific band with a proposed molecular weight of 32 to 35 kD was detected by G247-4 (Figure 1D). Neither NOK-1 nor mAb 33 stained rCD95-L even after 1 h of exposure following the ECL reaction. On the other hand, NOK-1 completely neutralizes the cytotoxic activity of rCD95-L derived from Alexis (not shown) suggesting that NOK-1 specifically recognizes CD95-L. This means that the reducing conditions of the Western blot experiment together with the linker sequences of the recombinant protein may alter some CD95-L epitopes. g protein loaded, 45 min exposure time). Therefore, mAb 33 is highly sensitive in a Western blot assay and loading too much protein extract may saturate the ECL-reaction masking differences between low or high level expression of CD95-L.1,2 To further characterize G247-4, NOK-1 and mAb 33, the binding properties to recombinant CD95-L (rCD95-L) from Alexis (Grünberg, Germany) were evaluated. rCD95-L corresponds to the extracellular domain of human CD95-L and is fused at the N-terminus to a linker peptide and a tag. After SDS – PAGE and Western blotting, a specific band with a proposed molecular weight of 32 to 35 kD was detected by G247-4 (Figure 1D). Neither NOK-1 nor mAb 33 stained rCD95-L even after 1 h of exposure following the ECL reaction. On the other hand, NOK-1 completely neutralizes the cytotoxic activity of rCD95-L derived from Alexis (not shown) suggesting that NOK-1 specifically recognizes CD95-L. This means that the reducing conditions of the Western blot experiment together with the linker sequences of the recombinant protein may alter some CD95-L epitopes. To compare the binding capacities of G247-4, NOK-1 and mAb 33 under non-reducing and reducing conditions we performed an immunoprecipitation assay using supernatants from 293 cells and rCD95-L from Alexis. Supernatants or rCD95-L in cell culture medium were incubated with G247-4, NOK-1 or mAb 33 under non-reducing conditions followed by precipitation with Protein A sepharose (Sigma, Deisenhofen, Germany). The immunoprecipitates were divided into three equal portions and each of them was analyzed by Western blotting. Probing the membranes with either G247-4, NOK-1 or mAb 33 detected enhanced levels of soluble CD95-L protein in CD95-L-transfected 293 cells only (Figure 1E). Soluble CD95-L migrated at a molecular weight of about 42 kD which correspond to a glycosylated form.6 Thus, full length CD95-L lacking any linker sequences is detected under non-reducing and reducing conditions by G247-4, NOK-1 and mAb 33. Truncated CD95-L protein with linker sequences (Alexis) was also specifically bound by G247-4, NOK-1 or mAb 33 under the non-reducing immunoprecipitation conditions since G247-4 stained a band with the proposed molecular weight. In contrast, neither NOK-1 nor mAb 33 detected immunoprecipitated rCD95-L (Alexis) after Western blotting. These data clearly demonstrate that under reducing conditions linker peptide and tag of rCD95-L mask epitopes which are required for the binding of NOK-1 and mAb 33. To investigate the functionality of mAb 33, G247-4 and NOK-1 in FACS analysis surface expression of CD95-L in the transfected 293 cells was examined. A species- and isotope-matched antibody was used as control. All of the CD95-L antibodies gave an enhanced staining in CD95-L overexpressing cells although with different affinities since the shift was highest using NOK-1 and lowest using mAb 33 (Figure 1F). On the basis of these results we conclude that mAb 33 is suited for analyzing natural CD95-L protein by Western blot an immunoprecipitation. mAb 33 is less sensitive in detecting surface expression CD95-L by flow cytometry. mAb 33 specifically binds rCD95-L from Alexis only in its native form e.g. in immunoprecipitation or neutralization assays but not in the denatured form e.g. in Western blot analysis.
|
Figure 1 Analysis of CD95-L by RT – PCR, Western blot and Supernatant-transfer-assay (A) 293 cells stably transfected with vector encoding the cDNA for full length CD95-L (CD95-L) or empty vector (CO) were examined by RT – PCR for expression of the CD95-L and the 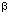 -ACTIN gene. Diminished -ACTIN gene. Diminished 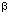 -ACTIN amounts in CD95-L overexpressing cells reflect the high spontaneous apoptosis rate in these cells. (B) Supernatant form 293 cells was added in a 1 : 1 ratio to JURKAT cells in the presence or absence of NOK-1 (25 -ACTIN amounts in CD95-L overexpressing cells reflect the high spontaneous apoptosis rate in these cells. (B) Supernatant form 293 cells was added in a 1 : 1 ratio to JURKAT cells in the presence or absence of NOK-1 (25  g/ml) or F(ab)2 fragment (1 g/ml) or F(ab)2 fragment (1  g/ml). Death was determined 24 h (grey bars) or 48 h (black bars) later by FACS using FSC/SSC analysis. (C) Protein extracts from 293 cells were examined by Western blot using G247-4, NOK-1 or mAb 33. The sizes of a molecular weight marker in kD are indicated on the left. The predicted size of 37 kD for CD95-L is marked by arrows. Ponceau red staining confirmed equal protein loading. (D) Recombinant CD95-L protein (rCD95-L) from Alexis was detected by Western blot analysis. The predicted size of 32 to 35 kD for rCD95-L is marked by an arrow. (E) For immunoprecipitation of CD95-L supernatants from 293 cells stably overexpressing empty vector or the cDNAs for CD95-L or TRAIL as well as rCD95-L (Alexis) diluted in medium were analyzed by immunoprecipitation with either NOK-1, G247-4 or mAb 33 together with protein A sepharose. Equal parts of each immunoprecipitate were examined in three different Western blot experiments using either G247-4, NOK-1 or mAb 33. The specific bands for soluble CD95-L or the heavy chain of the antibody (IgH) are marked by arrows. The size of a molecular weight marker in kD is indicated on the left. (F) Surface expression of CD95-L protein in stably transfected 293 cells was determined using mAb 33, G247-4, NOK-1 or isotype IgG1 control mAb by FACS-analysis and a representative out of five experiments is shown. CD95-L staining is represented by black profiles, empty vector staining by grey profiles and staining controls are marked by dotted lines. Prior to measurement of surface CD95-L expression by FACS-analysis cells were incubated for 12 h with 10-3 mM of the CD95-L specific metalloproteinase inhibitor KB8401 (Pharmingen, Hamburg, Germany). After harvesting, cells were stained with 10 g/ml). Death was determined 24 h (grey bars) or 48 h (black bars) later by FACS using FSC/SSC analysis. (C) Protein extracts from 293 cells were examined by Western blot using G247-4, NOK-1 or mAb 33. The sizes of a molecular weight marker in kD are indicated on the left. The predicted size of 37 kD for CD95-L is marked by arrows. Ponceau red staining confirmed equal protein loading. (D) Recombinant CD95-L protein (rCD95-L) from Alexis was detected by Western blot analysis. The predicted size of 32 to 35 kD for rCD95-L is marked by an arrow. (E) For immunoprecipitation of CD95-L supernatants from 293 cells stably overexpressing empty vector or the cDNAs for CD95-L or TRAIL as well as rCD95-L (Alexis) diluted in medium were analyzed by immunoprecipitation with either NOK-1, G247-4 or mAb 33 together with protein A sepharose. Equal parts of each immunoprecipitate were examined in three different Western blot experiments using either G247-4, NOK-1 or mAb 33. The specific bands for soluble CD95-L or the heavy chain of the antibody (IgH) are marked by arrows. The size of a molecular weight marker in kD is indicated on the left. (F) Surface expression of CD95-L protein in stably transfected 293 cells was determined using mAb 33, G247-4, NOK-1 or isotype IgG1 control mAb by FACS-analysis and a representative out of five experiments is shown. CD95-L staining is represented by black profiles, empty vector staining by grey profiles and staining controls are marked by dotted lines. Prior to measurement of surface CD95-L expression by FACS-analysis cells were incubated for 12 h with 10-3 mM of the CD95-L specific metalloproteinase inhibitor KB8401 (Pharmingen, Hamburg, Germany). After harvesting, cells were stained with 10  g/ml of either mAb 33, G247-4, NOK-1 or IgG1 isotype control mAb (Becton Dickinson, Heidelberg, Germany) followed by labeling with 20 g/ml of either mAb 33, G247-4, NOK-1 or IgG1 isotype control mAb (Becton Dickinson, Heidelberg, Germany) followed by labeling with 20  g/ml PE-coupled goat anti-mouse secondary Ab (Immunotech, Hamburg, Germany). Fixed cells were analyzed on a FACScan flow cytometer using the Cell Quest software (Becton Dickinson). An ELISA plate coated with immunogens at 2 g/ml PE-coupled goat anti-mouse secondary Ab (Immunotech, Hamburg, Germany). Fixed cells were analyzed on a FACScan flow cytometer using the Cell Quest software (Becton Dickinson). An ELISA plate coated with immunogens at 2  g/ml was incubated with dilutions of mAb 33 starting at 1 g/ml was incubated with dilutions of mAb 33 starting at 1  g/ml using a standard protocol g/ml using a standard protocol
|