|
|
|
Title
|
|
A phase I dose escalation study of multicyclic, dose-intensive chemotherapy with peripheral blood stem cell support for small cell lung cancer
|
 |
|
M Takahashi1, H Yoshizawa1, H Tanaka1, J Tanaka1, H Kagamu1, K Ito1, T Shimbo1, D Chou1, M Wakabayashi1, E Suzuki1, K Sakai2, M Arakawa1 & F Gejyo1
1Department of Medicine (II), Niigata University Medical School, Niigata, Japan
2Department of Radiology, Niigata University Medical School, Niigata, Japan
Correspondence to: H Yoshizawa, Department of Medicine (II), Niigata University Medical School, 1–757 Asahimachi-dori Niigata, 951–8510, Japan
|
|
Abstract
|
 |
A phase I dose-escalation study of multicyclic, ifosfamide, carboplatin, and etoposide (ICE) with sequential reinfusion of peripheral blood stem cells (PBSCs) was conducted to determine the maximum-tolerated dose (MTD) of ICE. Twenty-four patients with SCLC (LD: 6, ED: 18) were treated with ifosfamide (3000–9000 mg/m2, 24-h infusion), carboplatin (300–400 mg/m2), and etoposide (300 mg/m2) followed by subcutaneous filgrastim (75  g/day) from day 4 to the day of PBSC collection. PBSC were harvested when the WBC count reached 5 g/day) from day 4 to the day of PBSC collection. PBSC were harvested when the WBC count reached 5 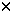 109/l. The leukapheresis product was cryopreserved and reinfused on day 4 of the next cycle, which was started 48 h after the last PBSC collection. The ifosfamide dose was escalated as follows: 3000 mg/m2 (level 1), 5000 mg/m2 (level 2), 7000 mg/m2 (level 3), 9000 mg/m2 (level 4). Patients with LD were treated with concurrent radiotherapy at 1.5 Gy twice daily for the initial 3 weeks to a total dose of 45 Gy and MTD, defined separately. Patients were evaluated for hematologic and non-hematologic toxicity, actual dose intensities, as well as response to therapy. The maximum-tolerated dose (MTD) was defined as the dose level at which more than 5 days of grade 4 myelo- suppression or non-hematologic toxicity greater than grade 3 developed in two thirds of the patients. For ED cases, MTD was level 4 and the recommended dose of ifosfamide was 7000 mg/m2. For LD cases, the recommended dose of ifosfamide was 5000 mg/m2. The dose limiting toxicity of multicyclic ICE was hemato- logic toxicity and CNS toxicity which manifested as ataxia. Tumor responses were seen in all patients, with 14 patients showing a complete response. The actual total dose-intensity at the recommended dose level was 2.2 and 1.74, for ED and LD, respectively, compared with previously reported ICE regimens. PBSC support for dose-intensive ICE regimen permitted dose escalation of ifosfamide with a mean interval of 16–17 days. We conclude that this regimen is well tolerated, with acceptable hematological and non-hematological toxicity. Bone Marrow Transplantation (2000) 25, 5–11. 109/l. The leukapheresis product was cryopreserved and reinfused on day 4 of the next cycle, which was started 48 h after the last PBSC collection. The ifosfamide dose was escalated as follows: 3000 mg/m2 (level 1), 5000 mg/m2 (level 2), 7000 mg/m2 (level 3), 9000 mg/m2 (level 4). Patients with LD were treated with concurrent radiotherapy at 1.5 Gy twice daily for the initial 3 weeks to a total dose of 45 Gy and MTD, defined separately. Patients were evaluated for hematologic and non-hematologic toxicity, actual dose intensities, as well as response to therapy. The maximum-tolerated dose (MTD) was defined as the dose level at which more than 5 days of grade 4 myelo- suppression or non-hematologic toxicity greater than grade 3 developed in two thirds of the patients. For ED cases, MTD was level 4 and the recommended dose of ifosfamide was 7000 mg/m2. For LD cases, the recommended dose of ifosfamide was 5000 mg/m2. The dose limiting toxicity of multicyclic ICE was hemato- logic toxicity and CNS toxicity which manifested as ataxia. Tumor responses were seen in all patients, with 14 patients showing a complete response. The actual total dose-intensity at the recommended dose level was 2.2 and 1.74, for ED and LD, respectively, compared with previously reported ICE regimens. PBSC support for dose-intensive ICE regimen permitted dose escalation of ifosfamide with a mean interval of 16–17 days. We conclude that this regimen is well tolerated, with acceptable hematological and non-hematological toxicity. Bone Marrow Transplantation (2000) 25, 5–11.
|
 |
| Keywords |
 |
|
small cell lung cancer; dose intensive chemotherapy; granulocyte colony-stimulating factor; hematopoietic stem cell transplantation; leukapheresis
|
 |
|
Received 2 November 1998; Accepted 15 April 1999

©
Macmillan Publishers Ltd 2000
|
|
|