|
N Layios, E Van Den Neste, E Jost, V Deneys, JM Scheiff & A Ferrant
Service d'Hématologie et de Biologie Hématologique, Cliniques Universitaires Saint-Luc, Université Catholique de Louvain, Brussels, Belgium
Correspondence to: E Van Den Neste, Service d'Hématologie, Cliniques Universitaires Saint-Luc, 10 av. Hippocrate, 1200 Brussels, Belgium; Fax: 32 2 7648959
|
TO THE EDITOR Rituximab (Mabthera; Roche, Brussels, Belgium) has demonstrated activity in the treatment of non-Hodgkin's lymphoma (NHL) of B cell origin.1 This antibody, which binds specifically to the CD20 antigen, a pan B cell marker, can deplete B cells through complement- and antibody-dependent cellular cytotoxicity. Rituximab is presumably active in Waldenström's macroglobulinemia (WM) because of the elimination of either CD20+ clonotypic precursor B cells, or CD20+ plasma cells, which have both been detected in most WM patients.2 The same rationale could apply to other immunoglobulin-mediated diseases of B lymphocytes, such as cold agglutinin disease (CAD) or cryoglobulinemia, which could also theoretically benefit from CD20 targeted therapy. We hereby describe a patient with refractory CAD who went into remission after therapy with Rituximab. A 67-year-old patient presented in April 1997 for acrocyanosis, weakness and dyspnoea. Her medical history included the diagnosis of an indolent NHL of the bone marrow (BM) in 1993, with secondary CAD. She had received chlorambucil (CLB) daily (5 mg/d) from January 1993 until April 1996 with resolution of signs of hemolysis. There were no enlarged lymph nodes. There was no hepatosplenomegaly. Laboratory studies showed a hemoglobin of 7.6 g/dl, a hematocrit of 23%, and a reticulocyte count of 66 000/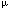 l. The white blood cell count was 1770/ l. The white blood cell count was 1770/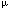 l with 51% neutrophils, 32% lymphocytes, and no abnormal forms. The platelet count was 37 000/ l with 51% neutrophils, 32% lymphocytes, and no abnormal forms. The platelet count was 37 000/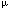 l. LDH were at 610 UI/l and total bilirubin was 1.7 mg/dl. Haptoglobin level was <12 mg/dl. The direct antiglobulin test was positive with IgM and complement. At elution, a monoclonal IgM with anti-I cold-agglutinin activity was found. BM analysis showed a diffuse and nodular lympho-plasmocytoid infiltrate (52% of total cellularity) with focal reticulinic fibrosis, together with erythroid hyperplasia and discrete dyserythropoiesis. Megakaryocytes were at the lower normal limit. Clonal rearrangement of the immunoglobulin heavy chain gene was found. The karyotype was normal on 10 mitoses. Whole-body CT scan was normal. l. LDH were at 610 UI/l and total bilirubin was 1.7 mg/dl. Haptoglobin level was <12 mg/dl. The direct antiglobulin test was positive with IgM and complement. At elution, a monoclonal IgM with anti-I cold-agglutinin activity was found. BM analysis showed a diffuse and nodular lympho-plasmocytoid infiltrate (52% of total cellularity) with focal reticulinic fibrosis, together with erythroid hyperplasia and discrete dyserythropoiesis. Megakaryocytes were at the lower normal limit. Clonal rearrangement of the immunoglobulin heavy chain gene was found. The karyotype was normal on 10 mitoses. Whole-body CT scan was normal. Because of pancytopenia, corticosteroid therapy was preferred to resuming CLB. She received sequential dexamethasone (20 mg/d for 4 days, 3 cycles per month during 2 months, followed by 1 cycle per month for 3 months), which was relayed by oral methylprednisolone. The transfusion need remained at 2 units of RBC every 3 weeks. In October 1997, there was a flare up of hemolysis, which led to the initiation of plasmapheresis together with 3 weekly doses of vincristine (1 mg each). A slight improvement was observed, as evidenced by a rise in haptoglobin and hemoglobin levels, without influence on the transfusion rate. Cyclophosphamide (two doses of 1 g each, at 4 weeks interval) did not improve the hemolysis. A BM examination was performed in August 1998 and proved to be similar to the initial one. Immunophenotyping showed monoclonal kappa light chain B lymphocytes expressing the CD20 antigen. High-dose corticosteroid therapy (methylprednisolone 1 g/6 week) was again attempted, along with plasmapheresis and vincristine, without benefit. Reticulocyte counts above 150 000/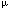 l were regularly observed throughout the evolution. In November 1998, the patient's hemolytic process was still active as shown by LDH and total bilirubin levels of 843 UI/l and 1.6 mg/dl, respectively, and haptoglobin <12 mg/dl. The IgM level was 409 mg/dl. The patient was included in a protocol comprising induction with Rituximab and maintenance with interferon- l were regularly observed throughout the evolution. In November 1998, the patient's hemolytic process was still active as shown by LDH and total bilirubin levels of 843 UI/l and 1.6 mg/dl, respectively, and haptoglobin <12 mg/dl. The IgM level was 409 mg/dl. The patient was included in a protocol comprising induction with Rituximab and maintenance with interferon- 2a (INF 2a (INF , Roferon; Roche) for the treatment of indolent NHL. She received 4 weekly doses of Rituximab (375 mg/m2 each), with good tolerance. No steroids were given. INF , Roferon; Roche) for the treatment of indolent NHL. She received 4 weekly doses of Rituximab (375 mg/m2 each), with good tolerance. No steroids were given. INF was initiated 2 weeks after the last dose of Rituximab at the dose of 4.5 was initiated 2 weeks after the last dose of Rituximab at the dose of 4.5  106 units, three times weekly, and continued for 16 weeks. Two months after starting Rituximab, LDH and total bilirubin had fallen to 412 UI/lL and 0.9 mg/dl, respectively. From then, the patient remained free of hemolysis for 7 months. Haptoglobin level and IgM component were 174 mg/dl and 59 mg/dl, respectively, in June 1999, when the patient was hospitalized for an epidural tumor (L5-S1) complicated by neurological compression. BM examination showed a normal cellularity, discrete reticulinic fibrosis, and no lymphomatous infiltration. There was no B cell monoclonality by flow cytometry analysis or Southern blotting. The tumor was thought to be lymphomatous, but no biopsy was performed because of thrombocytopenia. Radiotherapy was delivered, leading to a good response. In August 1999, hemolysis recurred severely and continued unabated despite corticosteroids and danazol. The patient died in February 2000 from a stroke. 106 units, three times weekly, and continued for 16 weeks. Two months after starting Rituximab, LDH and total bilirubin had fallen to 412 UI/lL and 0.9 mg/dl, respectively. From then, the patient remained free of hemolysis for 7 months. Haptoglobin level and IgM component were 174 mg/dl and 59 mg/dl, respectively, in June 1999, when the patient was hospitalized for an epidural tumor (L5-S1) complicated by neurological compression. BM examination showed a normal cellularity, discrete reticulinic fibrosis, and no lymphomatous infiltration. There was no B cell monoclonality by flow cytometry analysis or Southern blotting. The tumor was thought to be lymphomatous, but no biopsy was performed because of thrombocytopenia. Radiotherapy was delivered, leading to a good response. In August 1999, hemolysis recurred severely and continued unabated despite corticosteroids and danazol. The patient died in February 2000 from a stroke. This patient had an indolent clonal lymphoproliferative disease limited to the BM, a seric monoclonal IgM component with cold agglutinin activity, and severe hemolysis poorly responsive or refractory to prevention to cold exposure, corticosteroids, plasmapheresis, vincristine and high-dose cyclophosphamide. A lasting remission of hemolysis was achieved after Rituximab. Since hemolysis improved during Rituximab therapy, and went into remission after only six doses of INF , it is unlikely that INF , it is unlikely that INF played a role in inducing remission, although it may have contributed to its maintenance through an effect on the underlying NHL. Yet, the documented efficacy of INF played a role in inducing remission, although it may have contributed to its maintenance through an effect on the underlying NHL. Yet, the documented efficacy of INF in CAD is very limited.3 in CAD is very limited.3 A similar effect of Rituximab, in combination with corticosteroids and cyclophosphamide, has been reported in a patient with CAD secondary to NHL.4 Significant improvement has also been shown in a patient with refractory type II cryoglobulinemia after Rituximab therapy.5 Finally, B cell depletion with Rituximab also proved to be effective in IgM-related polyneuropathies, as shown by clinical improvement and decrease in serum autoantibodies titers.6 These observations encourage studies testing Rituximab in diseases characterized by the production of autoantibodies. In patients in whom a lymphoproliferative disorder is associated, the addition of interferons might prove useful since cytokines may modulate some of the immune effectors, such as antibody-dependent cellular toxicity or CD20 expression, involved in the cytotoxic effects of monoclonal antibodies.7,8 In this situation, the respective schedules of administration of these two drugs remain to be determined.
|