|
JA Snowden1,2, I Nivison-Smith1, K Atkinson1, K Fay1, A Concannon1, A Dodds1, S Milliken1 & J Biggs1
1Department of Haematology, St Vincent’s Hospital, Sydney, NSW 2010, Australia
2Correspondence: Dept of Haematology, Leicester Royal Infirmary, Leicester, LE1 5WW, UK
|
Compared with allogeneic bone marrow transplantation (BMT), allogeneic peripheral blood progenitor transplantation (PBPCT) is associated with a shortened time to engraftment and a similar incidence of acute graft-versus-host disease (GVHD).1 However, at present it is unclear whether there is an excess of chronic GVHD1,2,3 or perhaps most importantly, any survival advantage. A recent IBMTR analysis has shown that patients surviving beyond 2 years after BMT have a high probability of long-term survival (ie 89%).4 In order to evaluate our experience at St Vincent’s Hospital, Sydney, we have compared long-term follow-up data (ie longer than 2 years) from the first 16 patients to undergo allogeneic PBPCT with rigorously matched controls undergoing allogeneic BMT with an otherwise identical protocol. Patients were aged between 16 and 57 years and were receiving their first allogeneic transplant following conditioning with a variety of preparative regimens. HLA-A, -B, -DR-identical, mixed lymphocyte culture non-reactive sibling donors were mobilised with filgrastim 10  g/kg/day. Leukapheresis was commenced on day 5 and continued until the target minimum of 4.0 g/kg/day. Leukapheresis was commenced on day 5 and continued until the target minimum of 4.0 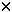 106 CD34+ cells per kg recipient weight were collected. GVHD prophylaxis was intravenous cyclosporin A 3 mg/kg/day until bowel function was normal, when oral cyclosporin 12.5 mg/kg/day in two divided doses was substituted, and methotrexate which was given according to our departmental protocol5 at a dose of 7.5 mg/m2 on days 1, 3, 6 and 11 post transplant. Day 11 methotrexate was omitted in patients who had severe mucositis. Supportive care was with a standardised protocol which included prophylaxis with ganciclovir, fluconazole, cotrimoxazole and heparin. 106 CD34+ cells per kg recipient weight were collected. GVHD prophylaxis was intravenous cyclosporin A 3 mg/kg/day until bowel function was normal, when oral cyclosporin 12.5 mg/kg/day in two divided doses was substituted, and methotrexate which was given according to our departmental protocol5 at a dose of 7.5 mg/m2 on days 1, 3, 6 and 11 post transplant. Day 11 methotrexate was omitted in patients who had severe mucositis. Supportive care was with a standardised protocol which included prophylaxis with ganciclovir, fluconazole, cotrimoxazole and heparin. For each of the 16 PBPCT patients enrolled between February and December 1996, a matched historical control patient, who had undergone BMT between June 1991 and November 1995, was carefully selected. PBPCT and BMT groups were therefore matched for diagnosis and disease stage at the time of transplant (nine acute leukaemia, one chronic myeloid leukaemia, two non-Hodgkin’s lymphoma, two myelodysplastic syndrome, one severe aplastic anaemia and one thalassaemia major in each group), patient age (median 33.5 vs 35 years) and donor age (median 33 vs 35 years), patient sex (12M:4F vs 11M:5F), female donor parity (median 2 vs 2.5), donor–recipient sex match (9 vs 7 sex matched, 7 vs 9 sex mismatched), the number of post-transplant methotrexate doses administered (median 4 vs 3) and preparative regimen (12 BuCy, 2 Cy/TBI, 1 Cy/ATG, 1 BuCyMel vs 13 BuCy, 1 Cy/TBI, 1 BuCyMel, 1 Cy only), respectively. All historical control patients had received prophylaxis for GVHD, infection and veno-occlusive disease (VOD) according to the same institutional protocol as PBPCT patients. Analysis was performed in April 1999 with a median observation time post transplant of 695 days for the PBPCT group and 1629 days for the control group. Minimum follow-up time for the surviving PBPCT patients was 895 days. Overall survival and the incidence of GVHD were compared using Kaplan–Meier product limit estimates or chi-squared tests. Differences between groups for other outcome parameters were assessed using Mann–Whitney U tests. The median time post transplant to a platelet count of 20 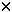 109/l was 14.5 days for PBPCT and 21 days for controls (P = 0.04). The median time post transplant to a platelet count of 50 109/l was 14.5 days for PBPCT and 21 days for controls (P = 0.04). The median time post transplant to a platelet count of 50 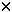 109/l was 17.5 days for PBPCT and 33.5 days for controls (P = 0.002). The median time post transplant to a neutrophil count of 0.5 109/l was 17.5 days for PBPCT and 33.5 days for controls (P = 0.002). The median time post transplant to a neutrophil count of 0.5 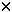 109/l was 14 days for PBPCT and 16.5 days for controls (P = 0.07). 109/l was 14 days for PBPCT and 16.5 days for controls (P = 0.07). Survival to day +100 post transplant was 13/16 (81%) in the PBPCT group and 14/16 (88%) in the BMT controls, with actuarial incidence of transplant-related mortality of 12.5% at 100 days for both groups (P = 0.9). In the PBPCT group there was one death from cerebral haemorrhage, one from VOD and one from relapse during the first 100 days post transplant and seven deaths thereafter (three chronic GVHD, two relapse, one diffuse alveolar damage syndrome, one pneumonia) ie 10 deaths in total (62.5%). In the control group there were two deaths during the first 100 days post transplant, both from VOD, and four thereafter (one chronic GVHD, two relapse, one sepsis) i.e. six deaths in total (37.5%). The difference in survival probabilities between PBPCT and BMT groups was not significant (P = 0.2). In patients surviving beyond day +21, the incidence of acute GVHD at day +100 post transplant was 69% for PBPCT and 75% for BMT (P = 0.8). Differences between total days of acute GVHD and the GVHD score (calculated by summating the figures produced from multiplying each grade of acute GVHD experienced by the number of days at that grade) were also not statistically significant. The incidence of acute GVHD of grade II or higher at day 100 post transplant was 31% in both groups (P = 0.99). Analysis of chronic GVHD (defined as clinical GVHD occurring after day +100, Table 1) shows increased incidence in the PBPCT group (P = 0.02), occurring in all evaluable patients in this group. The increase is observed in clinically extensive chronic GVHD (P = 0.03) and, when analysed by body distribution, chronic GVHD of the eyes and/or mouth (P = 0.02). There were three deaths due to chronic GVHD in the PBPCT groups compared with one in the BMT control group. In conclusion, although numbers are relatively small, this carefully matched retrospective analysis is supportive of an increased incidence of chronic GVHD with no apparent long-term survival benefit compared with BMT. The influence of methotrexate in GVHD prophylaxis has been previously discussed as a factor in explaining the conflicting results in previous studies,1 and this may be relevant in the present study where a reduced dose was used.5 Prospective randomised controlled trials are now in progress which should provide more definitive data. Analysis should account not only for the incidence of chronic GVHD generally but also for the pattern of organ involvement which may be significant in influencing overall survival and quality of life following allogeneic PBPCT. Acknowledgements
We thank the Arrow Foundation for their support.
|