Circulating peripheral blood progenitors are present in low numbers during steady-state hematopoiesis in healthy individuals,1 but are increased by the administration of growth factors, and can be harvested for transplantation.2 Following initial reports of the use of rhG-CSF-mobilized syngeneic or allogeneic peripheral blood stem cells (PBSC) for transplantation,3,4 there has been a dramatic increase in their use. Although the short-term adverse effects of rhG-CSF are well known5,6,7 and manageable, the long-term follow-up and the consequent safety of volunteer donors receiving growth factors for PBSC mobilization are not as well defined. Since G-CSF receptors are expressed in a variety of hematological malignancies, there has been concern by some that the administration of rhG-CSF to normal donors may induce hematopoietic disorders possibly including malignancies. The available data on long-term consequences of rhG-CSF administration in normal donors are limited to isolated reports. One study reported a 5 year follow-up of three donors8 and another reported a 3 year follow-up of nine donors.9 In a study of 19 donors mobilized on two separate occasions, 12–21 months apart, no differences in baseline complete blood counts were noted.10 The authors of that study, however, did not report on health problems, if any, occurring between mobilizations. In the present study, the results of long-term follow-up of 101 related donors who received short-term rhG-CSF to mobilize and collect either PBSCs or granulocytes 3 or more years ago are reported. Donors and methodsBetween November 1993 and April 1995, 101 normal donors received subcutaneous rhG-CSF prior to apheresis of either PBSC (n = 65) or granulocytes (n = 36). Donors were relatives of patients referred for allogeneic stem cell transplantation to the Fred Hutchinson Cancer Research Center, Seattle, USA or to the ‘Unita Trapianti, Divisione di Ematologia’ Ospedale ‘V Cervello’, Palermo, Italy. The general health criteria for donation of granulocytes were similar to policies for blood donation, including normal blood counts, negative hepatitis, and HIV serology, normal physical exam and electrocardiogram.11 In the case of stem cell donations, exceptions were made because these donors were relatives of the patients. As such, seven donors would have been excluded from routine blood donation due to a prior history of cancer (n = 2), myocardial infarction (n = 1), hypertension (n = 2), hepatitis C (n = 1), and Graves’ disease (n = 1). All donors signed an informed consent for donation PBSC or granulocytes and consented to the administration of rhG-CSF. Donor characteristics are shown in Table 1. There were 58 males and 43 females with a median age of 36.3 years (range 12.9–65.1). Fifteen were identical twins of their recipients. Twenty-six of the 36 granulocyte donors had previously donated bone marrow for transplantation. Donors were given rhG-CSF (Amgen, Thousand Oaks, CA, USA). Fifty-seven donors received 16  g/kg/day for a median of 6 days (range 4–8 days); 24 donors received 12 g/kg/day for a median of 6 days (range 4–8 days); 24 donors received 12  g/kg/day for a median of 6 days (range 3–15); eight donors received 10 g/kg/day for a median of 6 days (range 3–15); eight donors received 10  g/kg/day for a median of 5 days (range 4–8); five donors received 6 g/kg/day for a median of 5 days (range 4–8); five donors received 6  g/kg/day for a median of 11 days (range 9–14) and four received 5–3 g/kg/day for a median of 11 days (range 9–14) and four received 5–3  g/kg/day for a median of 13 days (range 12–14). Aphereses were performed on 1 to 2 consecutive days for PBSC donors, and 4–14 days for granulocyte donors. Venous access was obtained through venipuncture of both arms in 59 donors or through a central venous catheter in 42. Aphereses were performed with a continuous-flow blood cell separator (Cobe Laboratories, Lakewood, CO, USA or CS-3000 Plus, Fenwall Baxter Healthcare Corporation, Round Lake, IL, USA). All procedures were completed and short-term rhG-CSF side-effects were mild in all donors. At a time interval of 43.1 months (range 35.2–73.9), the donors were contacted by mail, telephone, facsimile or electronic mail to assess their current health status. All donors were asked to have a complete blood count (CBC) performed. The donors were questioned for the presence of health problems prior to the donation. Details of any new disease or worsening of pre-existing illnesses occurring after the rhG-CSF administration were recorded, as were pregnancies. The referring physicians were also called as needed to arrange blood testing or to provide further details of illnesses. g/kg/day for a median of 13 days (range 12–14). Aphereses were performed on 1 to 2 consecutive days for PBSC donors, and 4–14 days for granulocyte donors. Venous access was obtained through venipuncture of both arms in 59 donors or through a central venous catheter in 42. Aphereses were performed with a continuous-flow blood cell separator (Cobe Laboratories, Lakewood, CO, USA or CS-3000 Plus, Fenwall Baxter Healthcare Corporation, Round Lake, IL, USA). All procedures were completed and short-term rhG-CSF side-effects were mild in all donors. At a time interval of 43.1 months (range 35.2–73.9), the donors were contacted by mail, telephone, facsimile or electronic mail to assess their current health status. All donors were asked to have a complete blood count (CBC) performed. The donors were questioned for the presence of health problems prior to the donation. Details of any new disease or worsening of pre-existing illnesses occurring after the rhG-CSF administration were recorded, as were pregnancies. The referring physicians were also called as needed to arrange blood testing or to provide further details of illnesses. ResultsA total of 558 phone calls was made with a median of five calls (range 1–29) per donor. Of 101 donors receiving rhG-CSF more than 3 years ago, we were able to contact 95 (Table 2). At the time of the study 94 of the donors were alive and one had died of a drug overdose 15 months after receiving rhG-CSF. The events occurring before and after the rhG-CSF administration are summarized in Table 4. Two pregnancies occurred after and one during the rhG-CSF treatment despite a negative pregnancy test prior to the first dose of rhG-CSF. The three pregnancies resulted in two normal births with healthy children, but one pregnancy that occurred more than 2 years following the rhG-CSF resulted in a spontaneous abortion. One of the donors, who had a history of myocardial infarction, experienced angina during the apheresis. This donor underwent a coronary artery bypass graft which was complicated by a post-surgical Staphylococcus aureus infection. One month later he required a graft to the aorta due to the infection. Currently, he is well without cardiac symptoms. This donor was the only one requiring hospitalization during the rhG-CSF administration. Two of 94 evaluable donors had cancer diagnosed before rhG-CSF administration. One of the two, a 68-year-old woman, had a colon carcinoma surgically resected 1 year before rhG-CSF. The other, a 42-year-old woman, received high-dose chemotherapy and autologous bone marrow transplantation for breast cancer, 5 years before PBSC donation. Both were in complete remission at the time of donation and are still alive and well. One donor who was hepatitis C virus (HCV) positive before donation has developed abnormal liver function tests and is receiving alfa-interferon therapy. Two of 94 evaluable donors reported the occurrence of a neoplastic disease following rhG-CSF administration. One of the two, a 42-year-old woman, syngeneic to her recipient with breast cancer, was also diagnosed with stage I breast cancer 70 months after rhG-CSF administration. Her blood counts, before adjuvant chemotherapy, were within normal limits. The second, a 58-year-old man who donated PBSC for his HLA-identical sibling, was diagnosed with prostate cancer 15 months after rhG-CSF. One donor developed Parkinson’s disease 25 months following rhG-CSF administration. Another donor, who had Graves’ disease diagnosed prior to rhG-CSF administration, had a stroke 15 months after rhG-CSF. Her past history was consistent with Moyamoya disease. This is the same donor who had received rhG-CSF when 2–3 weeks pregnant, and subsequently delivered a healthy child, whose CBC was normal at birth. One donor, who noticed an enlarged supra-clavicular lymph node 38 months after rhG-CSF, underwent a biopsy which showed normal lymphoid architecture, and has subsequently done well with a further 18 months of follow-up without a recurrence of adenopathy. There were no other donors who developed signs or symptoms of hematological diseases. The CBC of 70 donors were collected (Table 3). Only one patient had a white blood cell count of 14 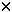 109/l, which was beyond the upper limit of normal. Granulocytes were also elevated in that patient at 10.3 109/l, which was beyond the upper limit of normal. Granulocytes were also elevated in that patient at 10.3 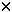 109/l. She was without symptoms at the time of this blood count, 5 years after receiving G-CSF. The donor declined to have a repeat CBC. 109/l. She was without symptoms at the time of this blood count, 5 years after receiving G-CSF. The donor declined to have a repeat CBC. DiscussionThis study was performed to evaluate the long-term safety of rhG-CSF given to healthy donors in order to mobilize either granulocytes or PBSC. At a median of 3.7 years of follow-up no important toxicities were observed that could be attributed to rh-CSF. Traditionally, the source of hematopoietic stem cells for allogeneic transplantation has been the bone marrow. Marrow harvesting is generally considered to be a safe procedure with minor morbidity. Following bone marrow harvesting up to 0.4% of donors experience life-threatening complications, usually related to general anesthesia. Complications have included severe hypotension, cardiac arrest, Staphylococcus aureus septicemia and osteomyelitis, severe weakness requiring hospitalization, bleeding requiring allogeneic blood transfusion, fever, and persistent pain due to injury of the skin, blood vessels, muscles and nerves.12,13,14 The use of autologous PBSC has increased dramatically in the past decade due to the larger progenitor cell yield, ease of collection, and excellent patient tolerance. The short-term adverse effects of rhG-CSF in normal donors are well documented but considered acceptable by most donors,5,6,7,15,16 while PBSC harvesting avoids the discomfort of marrow aspiration and the risks associated with general anesthesia. Recently, a spontaneous splenic rupture has been reported after rhG-CSF administration to a normal donor.17 It was unclear from the report whether the donor had an enlarged spleen or an underlying infection before rhG-CSF. Significant changes in serum biochemistry have also been reported, including rises in uric acid, lactate dehydrogenase and alkaline phosphatese.18,19 In spite of these effects, harvesting PBSCs has been reported to be preferred to harvesting bone marrow by some normal donors.20 While the short-term toxicities of rhG-CSF are known to be mild and mainly related to dose and time of administration,5 a concern remains about possible late effects in otherwise healthy individuals.21 In the present study, with a time interval of more than 3 years following rhG-CSF administration, the blood counts of the donors were found to be within normal limits, including hematocrit, platelet, leukocyte, granulocyte and lymphocyte counts, confirming that hematological modifications induced by rhG-CSF are short term and reversible. There are no studies on the use of rhG-CSF during pregnancy in healthy individuals and it is unknown whether rhG-CSF crosses the placenta.22 Neither the limited data in this article nor the data reported among women affected by severe chronic neutropenia receiving rhG-CSF during pregnancy23 are conclusive. We did not encounter donors with health problems which worsened after rhG-CSF, except for the donor who experienced angina during apheresis, following 16  g/kg/day rhG-CSF for 4 days. A brief report suggested a transient pre-thrombotic state in healthy donors receiving high-dose rhG-CSF.24 It is therefore possible that rhG-CSF influenced a recurrence of this donor’s cardiac symptoms. One donor who was pregnant during the rhG-CSF administration had a cerebrovascular accident 15 months later, which was thought to be related to a pre-rhG-CSF diagnosis of Moyamoya disease. g/kg/day rhG-CSF for 4 days. A brief report suggested a transient pre-thrombotic state in healthy donors receiving high-dose rhG-CSF.24 It is therefore possible that rhG-CSF influenced a recurrence of this donor’s cardiac symptoms. One donor who was pregnant during the rhG-CSF administration had a cerebrovascular accident 15 months later, which was thought to be related to a pre-rhG-CSF diagnosis of Moyamoya disease. One important question has been whether the short-term administration of rhG-CSF may induce leukemia or other malignancies in otherwise healthy donors. In vitro, rhG-CSF stimulates the proliferation and maturation of normal progenitor cells, myeloid leukemia cells, as well as non-hematopoietic cells. G-CSF receptors have also been found on the surface of other leukemic cells25 and solid tumor cell lines.26,27 Although the role of G-CSF surface receptors on these cells remains unclear, there is a theoretical concern that the administration of rhG-CSF may enhance leukemic or cancer cell proliferation. In a recent study of 531 patients with acute myeloblastic leukemia (AML), randomized to receive rhG-CSF following either induction or consolidation chemotherapy, a similar complete response rate and disease-free survival was observed in both arms, suggesting that rhG-CSF administration does not promote myeloid leukemia cell growth.28 This observation was confirmed in a Cancer and Leukemia Group B study of post-remission chemotherapy for AML in which rhG-CSF administration or placebo resulted in similar remission durations and survival.29 These observations have been confirmed in four studies of rhG-CSF use during remission induction for children and adults with ALL.30,31,32,33 It might be assumed from these studies that because rhG-CSF administration is safe in leukemic patients, it should also be safe in normal donors, even if the rhG-CSF receptor is expressed on quiescent neoplastic cells. The ability to detect an increase in leukemia among normal donors, however, is complicated by the observation that HLA-identical siblings of leukemia patients have been reported to carry a risk of developing leukemia which is somewhat higher than in the general population.26 Considerable data regarding the chronic use of rhG-CSF in patients affected by severe chronic neutropenia are available. None of the patients with cyclic or idiopathic neutropenia, receiving daily rhG-CSF developed AML or MDS. Conversely, leukemia occurred only in those patients with congenital neutropenia and the number of patients transforming each year during rhG-CSF administration remained constant.30 This suggests, at least, that rhG-CSF did not accelerate neoplastic transformation in these patients. Children with aplastic anemia have been reported to develop MDS/AML after treatment with rhG-CSF and immunosuppressive therapy,31 but MDS/AML have not been observed in children received rhG-CSF alone. In the current study 95 related donors have been surveyed. To date, the longest follow-up is 70.3 months. Among our donors, no hematological malignancies were observed. The two neoplastic events that occurred after rhG-CSF administration (a stage I breast cancer and a prostate cancer) represent the most common cancers in western countries. This finding is within the acceptable range of ‘cancer incidence’ in the normal adult population.32,33 Two of our donors had a diagnosis of cancer (colon carcinoma and breast cancer) prior to rhG-CSF administration. Both are in continuous complete remission at time intervals of 3.8 and 3.2 years after the rhG-CSF administration, and 4.8 and 8.2 years following the original diagnosis of malignancy. No other unusual diseases have been observed among donors in this study, which represents the longest follow-up of such donors to date. It will require much longer follow-up in a registry containing several thousand donors, however, to detect an increase in hematologic malignancies caused by rhG-CSF. Since the first allogeneic PBSC transplants were reported in 1992, there has been a dramatic increase in the use of these cells, harvested after mobilization with rhG-CSF. Already autologous transplants using PBSC have virtually replaced marrow as the preferred source of hematopoietic stem cells. It is therefore possible that there will be further increases in the number of PBSC allografts and along with it, increases in the number of normal individuals exposed to rhG-CSF. This limited study with a minimum follow-up of 3 years suggests that the administration rhG-CSF to normal donors appears safe and without obvious side-effects. Acknowledgements
This work was supported by Grants: CA 18029, CA 47748, CA 18221, CA 15704, CA 09515, the Jose Carreras Foundation Against Leukemia, and the Joseph Steiner Fund.
|