| Introduction Although many nonviral vector systems for gene transfer have been reported,1,2,3 only a few have been applied to practical therapeutic use. As far as conventional plasmid vectors are used, the transfection/expression efficiency by nonviral vectors has been relatively poor, particularly in in vivo gene transfer. To overcome this problem, we employed the Epstein–Barr virus (EBV)-based plasmid vectors instead of conventional plasmid vectors, and combined them with synthetic nonviral vectors.4,5,6 The EBV-based vector is a plasmid vector carrying EBV nuclear antigen 1 (EBNA1) gene and oriP from EBV genome.7 By binding to the oriP, the EBNA1 enables the plasmids to replicate and persist intracellularly for a long time. The EBNA1 also facilitates nuclear localization of plasmids, binding of plasmids to nuclear matrix, and transcriptional up-regulation. These functions bring the EBV plasmids high transfection/expression efficiency. The EBNA1 and the oriP are the only genetic elements from EBV, and no infectious viral particle can be produced by using the EBV-plasmid vector; the EBV-plasmid has to be combined with some gene delivery system (nonviral vector) to develop a nonviral system. We have previously demonstrated that by using the EBV-plasmid vectors, highly efficient transfection can be obtained in vitro into various cells of human origin.4,5,6,8,9 We have also shown that the polyamidoamine (PAMAM) dendrimer-mediated transfection of the EBV-plasmid vectors was quite effective in suicide gene transfer to tumor cells in vitro; when the herpes simplex virus type 1 thymidine kinase (HSV-1 tk) gene was transferred into three hepatocellular carcinoma cell lines, cells transfected with the EBV-based plasmid vector/PAMAM dendrimer (EBV/polyplex) exhibited two to three orders of magnitude higher susceptibility to ganciclovir (GCV) than those transfected with a conventional plasmid vector/ dendrimer.6 It has not been elucidated, however, whether the EBV/polyplex system can be applied to in vivo gene transfer, being advantageous over conventional nonviral systems. In this study, we examined the effectiveness of this nonviral system in an in vivo therapeutic model of Ewing’s sarcoma. Ewing’s sarcoma is a highly malignant, small round cell tumor that occurs mainly in children and young adults. Since conventional chemotherapy fails to provide sufficient effects resulting in poor prognosis, this tumor is a good candidate for suicide gene therapy. ResultsEfficient marker gene transfer in vitro into Ewing’s sarcoma cell lines by EBV-based plasmid vector/dendrimer We examined whether the EBV-based plasmid vector coupled with PAMAM dendrimer is an effective vector system to transfer exogenous genes in vitro. pSES. (an EBV-based plasmid vector carrying the (an EBV-based plasmid vector carrying the 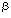 -gal gene) or pS. -gal gene) or pS. (a conventional plasmid vector with the (a conventional plasmid vector with the 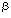 -gal gene) (Figure 1a) was transfected into Ewing’s sarcoma, A4573,10 and 3 days later, X-gal staining was performed. Approximately 20% of A4573 cells given pSES. -gal gene) (Figure 1a) was transfected into Ewing’s sarcoma, A4573,10 and 3 days later, X-gal staining was performed. Approximately 20% of A4573 cells given pSES. / dendrimer were strongly positive in a dense blue color, and up to 34% were positive including weakly positive cells in a pale blue color (Figure 2a). In contrast, only 5% of pS. / dendrimer were strongly positive in a dense blue color, and up to 34% were positive including weakly positive cells in a pale blue color (Figure 2a). In contrast, only 5% of pS. /dendrimer-transfected cells stained with X-gal (Figure 2b). Endogenous /dendrimer-transfected cells stained with X-gal (Figure 2b). Endogenous  -gal activity was not detected in A4573 cells by an X-gal staining experiment (data not shown). -gal activity was not detected in A4573 cells by an X-gal staining experiment (data not shown). The  -gal assay also showed that the -gal assay also showed that the  -gal activity in pSES. -gal activity in pSES. /dendrimer-transfected cells was 1.9- to three-fold higher than that in pS. /dendrimer-transfected cells was 1.9- to three-fold higher than that in pS. /dendrimer-transfected cells (Figure 1b). This ratio is not exactly the same as that obtained from X-gal staining. The discrepancy may be ascribed to the different sensitivities of the two assays. X-gal staining shows the transgene expression at single cell level, while the enzyme assay gives more quantitative data of cell population. Nevertheless, data of both assays confirmed the superiority of the EBV-plasmid to the conventional plasmid vector. Similar data were also obtained by transfecting another Ewing’s sarcoma cell line, KP-EWS-YI (Figure 1c). /dendrimer-transfected cells (Figure 1b). This ratio is not exactly the same as that obtained from X-gal staining. The discrepancy may be ascribed to the different sensitivities of the two assays. X-gal staining shows the transgene expression at single cell level, while the enzyme assay gives more quantitative data of cell population. Nevertheless, data of both assays confirmed the superiority of the EBV-plasmid to the conventional plasmid vector. Similar data were also obtained by transfecting another Ewing’s sarcoma cell line, KP-EWS-YI (Figure 1c). High susceptibility to GCV of Ewing’s sarcoma cells transfected with pSES.Tk/dendrimer in vitro Next, to verify the efficiency of EBV-based plasmid vector in suicide gene transfer, we assessed susceptibility to GCV of A4573 or KP-EWS-YI cells transfected with the HSV-1 tk gene. Cells transfected with pSES.Tk (an EBV-based plasmid vector carrying the HSV-1 tk gene) showed 100- to 300-fold higher susceptibility to GCV than those transfected with pS.Tk (a conventional plasmid vector with HSV-1 tk gene) (Figure 3a and b). The ID50 to GCV for pSES.Tk-transfected and pS.Tk-transfected A4573 cells were 3 and 300  M, respectively. The KP-EWS-YI cells transfected with pSES.Tk and pS.Tk showed ID50 values of 20 and 2000 M, respectively. The KP-EWS-YI cells transfected with pSES.Tk and pS.Tk showed ID50 values of 20 and 2000  M, respectively. M, respectively. Ewing’s sarcoma cells transfected with pSES.Tk/dendrimer were totally killed by GCV Cells transfected with pSES.Tk or pS.Tk were cultured with GCV at a concentration of 30  M, that is close to the serum concentration in patients intravenously administered with GCV for treatment.11 As shown in Figure 3c, A4573 cells transfected with pSES.Tk remarkably reduced in cell number, and virtually no cells survived until day 7. In contrast, more than 40% of pS.Tk-transfected cells survived in the culture. Similar data were also obtained with KP-EWS-YI cells (Figure 3d). M, that is close to the serum concentration in patients intravenously administered with GCV for treatment.11 As shown in Figure 3c, A4573 cells transfected with pSES.Tk remarkably reduced in cell number, and virtually no cells survived until day 7. In contrast, more than 40% of pS.Tk-transfected cells survived in the culture. Similar data were also obtained with KP-EWS-YI cells (Figure 3d). EBV/polyplex is effective in in vivo gene transfer into tumors To examine if the EBV-based plasmid vectors could be introduced into tumor cells in vivo by means of PAMAM dendrimer, 100  g of pSES. g of pSES. or pS. or pS. was coupled with 300 was coupled with 300  g of dendrimer and injected into tumors established in immuno-incompetent mice. Three days after the injection, mice were killed and tumors were excised for X-gal staining. In agreement with the results in vitro, g of dendrimer and injected into tumors established in immuno-incompetent mice. Three days after the injection, mice were killed and tumors were excised for X-gal staining. In agreement with the results in vitro,  -gal expression was found to be stronger in pSES. -gal expression was found to be stronger in pSES. / dendrimer-injected tumors than in pS. / dendrimer-injected tumors than in pS. /dendrimer- injected ones (Figure 4). As far as we tested, naked EBV-plasmid DNA was not effective in transducing genes in vivo (data not shown). /dendrimer- injected ones (Figure 4). As far as we tested, naked EBV-plasmid DNA was not effective in transducing genes in vivo (data not shown). Remarkable in vivo therapeutic effects by EBV/polyplex An in vivo therapeutic experiment was performed as follows: A4573 cells were inoculated subcutaneously into SCID mice, and 10 days later, pSES.Tk or pS.Tk/dendrimer was injected into the established tumors. Control tumors were injected with dendrimer alone, so that possible nonspecific effects by the macromolecule per se can be ruled out. After administering GCV for 14 days, we estimated the tumor volumes as well as survival of mice. Volumes of residual tumors injected with pSES.Tk/dendrimer were significantly smaller than those given pS.Tk/dendrimer injection (P < 0.05) (Figure 5a). The single-round treatment gave a significant therapeutic effect for over 40 days. Mice treated with pSES.Tk/dendrimer significantly improved in survival when compared with control mice (P < 0.02) or those treated with pS.Tk/dendrimer (P < 0.02) (Figure 5b). We hypothesized that repetitive treatment may result in a stronger therapeutic effect. An injection of pSES.Tk or pS.Tk/dendrimer was followed by six daily administrations of GCV, and these treatments were repeated four times. The pSES.Tk/dendrimer treatment induced drastic suppression of tumor growth and prolonged survival of mice, while pS.Tk/dendrimer treatment did not significantly influence tumor growth or longevity of the animals (Figure 6a and b). Repetitive injections with pSES.Tk/dendrimer resulted in stronger suppression of tumor growth and more prolonged survival of mice compared with the single injection with the same DNA/dendrimer complex (Figure 5a and b). In both experiments, all mice finally died of progressive tumors in the natural course. We could not observe any sign of inflammation in any tumor (data not shown). The growth of control tumors was more progressive in the repetitive treatment experiment (Figure 6a) than in the single treatment one (Figure 5a). The pS.Tk/dendrimer treatment could partially suppress mildly growing but not progressively growing tumors. The in vivo effectiveness of the EBV/polyplex was also confirmed with another tumor cell line, ie Huh7, a human hepatocellular carcinoma (HCC). Again, tumor growth was more intensively suppressed by intra-tumoral injection with pSES.Tk/dendrimer than that with pS.Tk/dendrimer (Figure 5c). DiscussionIn this study, we demonstrated that the EBV-based plasmid vector/PAMAM dendrimer is quite effective in suicide gene transfer not only in in vitro experiments but also in in vivo therapeutic models of mice implanted with Ewing’s sarcoma or HCC. Recently, the HSV-1 tk/GCV system has been applied not only to animal models but also to clinical trials for some malignant tumors in patients, such as glioblastoma, malignant melanoma, ovarian carcinoma and mesothelioma.12,13,14,15 Adenoviral and retroviral vectors have been frequently used to deliver the HSV-1 tk gene, while there have been few reports showing that a suicide gene was successfully transferred in vivo with a nonviral vector system. Four groups, including us, have reported that plasmid vectors carrying oriP but EBNA1 were effective in transferring the HSV-1 tk in vitro into EBV-associated lymphoma cells;5,16,17,18 these particular cancer cells strongly express endogenous EBNA1, allowing strong expression from oriP-bearing plasmids. The strategy, however, cannot be applied to common cancers, which do not express EBNA1. As far as we know, the present study is the first report showing that the EBV-episomal vectors carrying both oriP and EBNA1 are effective in suicide gene therapy of an EBNA1-negative cancer both in vitro and in vivo. We employed the PAMAM dendrimer as a delivery vehicle for the plasmid vectors. The PAMAM dendrimer is a branched spherical polymer, whose surface is positively charged by primary amino groups. Some groups have utilized this polymer for in vitro gene transfer of genetic materials into cultured cells.2,3,6,19,20,21 Qin et al22 reported that dendrimer can mediate efficient ex vivo transfer of plasmid DNA to murine cardiac graft. To our knowledge, our present study is the first report showing that PAMAM dendrimer is effective in in vivo gene transfer into tumors. Notably, in the present observations, the repetitive injection brought stronger suppression of tumor growth. As far as we noticed, the repetitive administration of pSES.Tk/dendrimer did not cause any toxic effects throughout the experiment. By means of the EBV/polyplex, sequential therapy could be possible and lead to more satisfying results. Although  -gal expression was seen in only approximately 34% of pSES. -gal expression was seen in only approximately 34% of pSES. -transfected A4573 cells in vitro (Figure 2a), tumor growth was remarkably suppressed by pSES.Tk/dendrimer and GCV treatment in vivo (Figure 5). This may be attributed to the bystander effect.23,24,25 When HSV-1 tk-positive and -negative cells were mixed at various ratios, as few as 10% of HSV-1 tk-positive cells could reportedly kill 100% of the mixed cell population.23 -transfected A4573 cells in vitro (Figure 2a), tumor growth was remarkably suppressed by pSES.Tk/dendrimer and GCV treatment in vivo (Figure 5). This may be attributed to the bystander effect.23,24,25 When HSV-1 tk-positive and -negative cells were mixed at various ratios, as few as 10% of HSV-1 tk-positive cells could reportedly kill 100% of the mixed cell population.23 In conclusion, the EBV-based plasmid vector coupled with PAMAM dendrimer is superior to the conventional plasmid vector/dendrimer, in terms of gene transfer efficiency as well as therapeutic effects both in vitro and in vivo. The present results obtained with the Ewing’s sarcoma and HCC cell lines are now being verified with other EBNA1-negative tumor cell lines. The EBV-based plasmid vectors may be potentially useful for other strategies of gene therapy and also applicable to various malignant tumors. Materials and methodsPlasmid vectors The plasmid vectors, pSES. , pSES.Tk, pS. , pSES.Tk, pS. and pS.Tk, are schematically illustrated in Figure 1a. Briefly, pSES. and pS.Tk, are schematically illustrated in Figure 1a. Briefly, pSES. contains the E. coli contains the E. coli 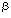 -gal gene driven by the SR -gal gene driven by the SR promoter, EBV oriP, EBV EBNA1 gene driven by the SR promoter, EBV oriP, EBV EBNA1 gene driven by the SR promoter, the ampicillin-resistant gene and the replication origin for E. coli. pSES.Tk is essentially the same as pSES. promoter, the ampicillin-resistant gene and the replication origin for E. coli. pSES.Tk is essentially the same as pSES. , except that pSES.Tk carries the HSV-1 tk gene instead of the , except that pSES.Tk carries the HSV-1 tk gene instead of the 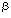 -gal gene. The other plasmids, pS. -gal gene. The other plasmids, pS. and pS.Tk, were constructed from pSES. and pS.Tk, were constructed from pSES. and pSES.Tk, respectively, by deleting SR and pSES.Tk, respectively, by deleting SR –EBNA1 and oriP.6 –EBNA1 and oriP.6 Cell lines A4573 and KP-EWS-YI cells were maintained in RPMI1640 medium (Gibco-BRL, Gaithersburg, MD, USA) supplemented with 100 U/ml penicillin, 100  g/ml streptomycin and 10% fetal bovine serum. Huh7 cells were maintained in Dulbecco’s modified Eagle’s medium (Gibco-BRL) supplemented with 100 U/ml penicillin, 100 g/ml streptomycin and 10% fetal bovine serum. Huh7 cells were maintained in Dulbecco’s modified Eagle’s medium (Gibco-BRL) supplemented with 100 U/ml penicillin, 100  g/ml streptomycin and 10% fetal bovine serum. g/ml streptomycin and 10% fetal bovine serum. In vitro gene transfer A4573 and KP-EWS-YI cells were plated in six-well plates (Falcon, Lincoln Park, NJ, USA). On the next day, 6  g of plasmid DNA was mixed with 30 g of plasmid DNA was mixed with 30  g of PAMAM dendrimer (Qiagen, Hilden, Germany). After 10 min of incubation at room temperature, the DNA/dendrimer complex was added into the plates. Cells were cultured at 37°C in 5% CO2/95% humidified air for 3 days and examined by X-gal staining, g of PAMAM dendrimer (Qiagen, Hilden, Germany). After 10 min of incubation at room temperature, the DNA/dendrimer complex was added into the plates. Cells were cultured at 37°C in 5% CO2/95% humidified air for 3 days and examined by X-gal staining,  -gal assay and Alamar blue assay. -gal assay and Alamar blue assay. X-gal staining of cultured cells Cells were fixed with 1% glutaraldehyde/PBS for 10 min, washed three times with PBS, and incubated in X-gal staining solution (0.05% (v/v) 5-bromo-4-chloro-3-indolyl- –D-galactoside (X-gal), 1 mM MgCl2, 150 mM NaCl, 3 mM K4(Fe(CN)6), 3 mM K3(Fe(CN)6), 60 mM Na2HPO4 and 0.1% Triton X-100). After incubation for 3 h at 37°C, the reaction was terminated by replacing the solution with 1 mM Na2-EDTA/PBS. –D-galactoside (X-gal), 1 mM MgCl2, 150 mM NaCl, 3 mM K4(Fe(CN)6), 3 mM K3(Fe(CN)6), 60 mM Na2HPO4 and 0.1% Triton X-100). After incubation for 3 h at 37°C, the reaction was terminated by replacing the solution with 1 mM Na2-EDTA/PBS. 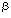 -Gal assay -Gal assay
Cells were scraped from plates, washed twice with PBS and resuspended in 50  l of Tris-HCl (pH 7.8). After freezing and thawing twice, the lysate was centrifuged at 14 000 g for 5 min. l of Tris-HCl (pH 7.8). After freezing and thawing twice, the lysate was centrifuged at 14 000 g for 5 min.  -Gal activity in the supernatant was assayed with a -Gal activity in the supernatant was assayed with a  -Gal assay kit (Invitrogen, San Diego, CA, USA) according to the manufacturer’s protocol. The optical density (OD) was measured at 420 nm (OD420). The activity was calculated as follows: -Gal assay kit (Invitrogen, San Diego, CA, USA) according to the manufacturer’s protocol. The optical density (OD) was measured at 420 nm (OD420). The activity was calculated as follows:  -gal units = (380 -gal units = (380 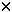 OD420)/t/mg protein, where t = incubation time in minutes. The protein concentration of the supernatant was assessed according to the method of Bradford. OD420)/t/mg protein, where t = incubation time in minutes. The protein concentration of the supernatant was assessed according to the method of Bradford. Alamar blue assay Susceptibility of cells to GCV was examined by an Alamar blue assay.5 Briefly, triplicate aliquots of cells transfected with pSES.Tk or pS.Tk were plated in 96-well flat-bottom microtiter plates (Falcon) (1  104 cells in 200 104 cells in 200  l of complete medium per well) and cultured in the presence or absence of various concentrations of GCV. As a control group, cells treated with dendrimer alone were also tested. After incubation for 72 h at 37°C in 5% CO2/95% humidified air, 10 l of complete medium per well) and cultured in the presence or absence of various concentrations of GCV. As a control group, cells treated with dendrimer alone were also tested. After incubation for 72 h at 37°C in 5% CO2/95% humidified air, 10  l of Alamar blue (Alamar Bioscience, Sacramento, CA, USA) was added. Cells were further cultured for 4 h, and the OD of each well was measured with a microplate reader, using test and reference wave lengths of 570 and 600 nm, respectively. The percentage of viable cells was calculated according to the following formula: % viable cells = (OD570–OD600) of GCV-treated cells/(OD570–OD600) of untreated cells. l of Alamar blue (Alamar Bioscience, Sacramento, CA, USA) was added. Cells were further cultured for 4 h, and the OD of each well was measured with a microplate reader, using test and reference wave lengths of 570 and 600 nm, respectively. The percentage of viable cells was calculated according to the following formula: % viable cells = (OD570–OD600) of GCV-treated cells/(OD570–OD600) of untreated cells. In vivo study with A4573 SCID mice were purchased from CLEA JAPAN (Osaka, Japan). Twenty-four hours before tumor cell inoculation, anti-asialo-GM1 antibody (Wako, Osaka, Japan) was intraperitoneally administered to 8- to 9-week-old SCID mice. Ten million A4573 cells were subcutaneously injected into the flanks of the mice (day 0). Within 10 days following the tumor cell injection, tumors of 2–3 mm in diameter developed. On day 10, 100  g of plasmid was coupled with 300 g of plasmid was coupled with 300  g of dendrimer as described above, and injected into established tumors. As a control, a group of mice was given intratumoral injection of PAMAM dendrimer alone. From the next day, intraperitoneal administration of 100 mg/kg of GCV was given daily for 14 days. In another experiment, plas- mid/dendrimer complex was injected into tumors, followed by daily administration of 100 mg/kg/day of GCV for 6 days, and these treatments were repeated four times. The diameters of tumors were scaled twice a week with a digital scalper. The tumor volume was calculated as follows: volume = a2 g of dendrimer as described above, and injected into established tumors. As a control, a group of mice was given intratumoral injection of PAMAM dendrimer alone. From the next day, intraperitoneal administration of 100 mg/kg of GCV was given daily for 14 days. In another experiment, plas- mid/dendrimer complex was injected into tumors, followed by daily administration of 100 mg/kg/day of GCV for 6 days, and these treatments were repeated four times. The diameters of tumors were scaled twice a week with a digital scalper. The tumor volume was calculated as follows: volume = a2 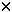 b/2 (mm3), where a = long diameter and b = short diameter. b/2 (mm3), where a = long diameter and b = short diameter. In vivo study with Huh7 Five million Huh7 cells were subcutaneously injected into the flanks of SCID mice as above (day 0). On day 20, 100  g of plasmid was coupled with 300 g of plasmid was coupled with 300  g of dendrimer, and injected into established tumors. As a control, a group of mice was given intratumoral injection with PAMAM dendrimer alone. From the next day, intraperitoneal administration of 100 mg/kg of GCV was given daily for 7 days. Tumor volume was calculated as above. g of dendrimer, and injected into established tumors. As a control, a group of mice was given intratumoral injection with PAMAM dendrimer alone. From the next day, intraperitoneal administration of 100 mg/kg of GCV was given daily for 7 days. Tumor volume was calculated as above. X-gal staining of tumors Mice were killed three days after plasmid/dendrimer complex injection. Tumors were excised and cut into pieces. Part of the tumor pieces was stained with X-gal as described previously. The other tumor pieces were frozen, cut with a cryostat into 10  m slices and subjected to X-gal staining. m slices and subjected to X-gal staining. Statistical evaluation For the  -gal assay and the GCV-susceptibility experiment, Fisher’s exact test was used. For animal studies, comparison of survival differences between groups was performed using the Wilcoxon test. -gal assay and the GCV-susceptibility experiment, Fisher’s exact test was used. For animal studies, comparison of survival differences between groups was performed using the Wilcoxon test. Acknowledgements
We thank F Hoffmann-La Roche Ltd (Basel, Switzerland) for kindly providing us with GCV. We also thank Ms Satoko Watanabe (Department of Microbiology, Kyoto Prefectural University of Medicine) for her excellent secretarial assistance. This research was supported by a grant-in-aid for scientific research from the Ministry of Education, Science, Sports and Culture, Japan, and the Japan Heart Foundation and IBM Japan Research Grant.
|