| Introduction Acute myeloid leukemia (AML) lends itself as a model disease for the evaluation of the clinically relevant mechanisms of drug resistance. Several mechanisms of multidrug resistance have previously been studied for their clinical relevance in AML (see Refs 1–3 for review). MDR1/P-glycoprotein expression was associated with poor response to induction chemotherapy and shorter survival,4,5,6,7,8 whereas expression of the multidrug resistance protein (MRP1) had no impact on clinical outcome.9,10 Expression of the lung resistance protein (LRP) was recently shown to correlate with lower response rates of induction chemotherapy and shorter survival by List et al11 and our group.12 Expression of the proto-oncogene bcl-2, which is involved in the regulation of apoptosis,13 indicated poor clinical outcome.14 To compare the clinical relevance of these mechanisms of drug resistance on a representative patient population, we studied four drug resistance factors (LRP, P-gp, MRP1, bcl-2) in addition to age and karyotype for their predictive value with regard to outcome of induction chemotherapy and their prognostic value with regard to survival in both univariate and multivariate analyses in 111 patients with de novo AML. Here we report the results of these studies. Patients and methodsPatients One hundred and eleven patients (49 female, 62 male) with de novo AML were included in this study after obtaining informed consent. Eighty of these patients had been included in a previous study on the clinical relevance of LRP.12 Patients were treated between January 1990 and February 1998. The characteristics of the patients are summarized in Table 1. Patients received one to two cycles of standard induction chemotherapy. Chemotherapy consisted of daunorubicin 45 mg/m2 daily on days 1–3 and cytarabine 200 mg/m2 daily on days 1–7 (DA protocol) in 25 patients and additional etoposide 100 mg/m2 daily on days 1–5 (DAE protocol) in 71 patients. Four patients were treated with idarubicin plus cytarabine (IA protocol). Eight patients with FAB subtype M3 received all-trans retinoic acid (ATRA) followed by chemotherapy. Three patients received intermediate-dose cytarabine followed by DAE as second induction chemotherapy cycle. Response to induction chemotherapy was assessed according to standard criteria. Seventy-five of 78 patients in complete remission received consolidation therapy with conventional-dose cytarabine (n = 29) or high-dose cytarabine (n = 46). Nineteen patients underwent bone marrow transplantation. Immunocytochemistry Immunocytochemistry was performed as described.9,12 Briefly, cells were fixed in cold acetone and incubated for 2 h with the primary monoclonal antibodies described below. Antibody binding was detected by the avidin–biotin– peroxidase method. Staining of at least 200 leukemic cells was evaluated independently by three investigators who had no previous knowledge of clinical data of the patients. Because cut-off levels ranging from 5%7,15,16 to 20%6,17,18,19,20 have been described for P-gp and from 5%21 to 20%9 for MRP1, we decided to divide P-gp and MRP1 expression into three categories (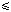 5%, 6–20%, >20% staining cells). 5%, 6–20%, >20% staining cells). LRP: The LRP-56 antibody was used (Alexis, Läufelfingen, Switzerland).12 Dependent on the percentage of staining cells, LRP expression was divided into negative (<5% staining cells) and positive (5%) expression. The small-cell lung cancer cell line SW1573 and its drug-resistant variant SW1573/2R120 (both cell lines provided by Dr RJ Scheper) were used as negative and positive controls, respectively.22 P-gp: The C219 (Alexis) antibody was used.9,12 Dependent on the percentage of staining cells, P-gp expression was divided into low (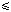 5%), intermediate (6–20%) and high (>20%) expression. Drug-sensitive KB-3-1 and multidrug-resistant KB-8-5 cells (provided by Drs I Pastan and MM Gottesman, National Cancer Institute, Bethesda, MD, USA) were used as negative and positive controls, respectively.4 5%), intermediate (6–20%) and high (>20%) expression. Drug-sensitive KB-3-1 and multidrug-resistant KB-8-5 cells (provided by Drs I Pastan and MM Gottesman, National Cancer Institute, Bethesda, MD, USA) were used as negative and positive controls, respectively.4 MRP1: A combination of QCRL-1/QCRL-3 antibodies was used.9 Dependent on the percentage of staining cells, MRP1 expression was divided into low (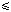 5%), intermediate (6–20%) and high (>20%) expression. C1 cells were used as negative controls and T5 cells as positive controls (provided by Drs SPC Cole and RG Deeley, Queen's University, Kingston, Canada).23 5%), intermediate (6–20%) and high (>20%) expression. C1 cells were used as negative controls and T5 cells as positive controls (provided by Drs SPC Cole and RG Deeley, Queen's University, Kingston, Canada).23 bcl-2: The bcl-2 antibody was used (Neomarkers, Fremont, CA, USA). Dependent on the percentage of staining cells, bcl-2 expression was divided into negative ( antibody was used (Neomarkers, Fremont, CA, USA). Dependent on the percentage of staining cells, bcl-2 expression was divided into negative (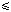 20%) and positive (>20%). 20%) and positive (>20%). To ensure specificity of staining and to overcome inter-assay variability, several controls were performed. These controls included negative and positive control cell lines. In addition, experiments without monoclonal antibodies were performed as negative controls in all cases, and controls with irrelevant isotype-specific antibodies were done in some cases. There were no differences in staining between the irrelevant isotype-specific antibodies and the negative controls without any primary antibody. Finally, we analyzed selected samples (at least one sample per expression category for C219, QCRL-1/QCRL-3, LRP-56 and bcl2- ) at least three times in order to confirm the reproducibility of our immunocytochemical assay. In these cases, the repetitions gave consistent results with identical categories of expression for all drug resistance factors (data not shown). ) at least three times in order to confirm the reproducibility of our immunocytochemical assay. In these cases, the repetitions gave consistent results with identical categories of expression for all drug resistance factors (data not shown). Karyotype analysis Good prognosis karyotype included inv(16), t(8;21) or t(15;17). Poor prognosis included del5q or -5, del7q or -7, +8, inv(3) or t(3;3), and complex aberrations. Cytogenetic abnormalities others than the ones described above or normal karyotype were regarded as indicators of intermediate prognosis. Statistical analysis Associations between clinical parameters and drug resistance factors were assessed by chi-squared tests. Survival probabilities were calculated with the product limit method according to Kaplan–Meier.24 Overall survival time was defined as the period between the time of diagnosis and the time of death. Disease-free survival time was defined as the period between the time when complete remission was first demonstrated and the time of relapse or death. Survival times of patients still alive or patients who underwent bone marrow transplantation were censored with the last follow-up date or transplantation date, respectively. Logistic regression models and Cox proportional hazards regression models25 were used to assess the independent effects of covariables on complete remission and survival. All P values are results of two-sided tests. The SAS statistical software system (SAS Institute, Cary, NC, USA, 1990) was used for calculations. ResultsExpression of drug resistance factors One hundred and eleven patients with de novo AML were studied and their characteristics are summarized in Table 1. Drug resistance factors were determined in leukemic cells at the time of diagnosis (Table 2). LRP expression was negative in 66% and positive in 34% of the patients. Low, intermediate and high expression of P-gp was seen in 59%, 29% and 12% of the patients, respectively, and of MRP1 in 30%, 47% and 23%. Expression of bcl-2 was negative in 46% and positive in 54% of the patients. Next, the drug resistance factors were assessed for potential associations with either each other or clinical parameters. LRP positivity was associated with higher white blood cell count (P = 0.0003), higher lactate dehydrogenase serum levels (P = 0.03), and increased expression of P-gp, MRP1 and bcl-2 (Table 3 and data not shown). P-gp correlated with expression of MRP1 and bcl-2 (Table 3), and was less frequent in both certain FAB subtypes (M3, M4Eo) (P = 0.03) and good karyotype (P = 0.03) (data not shown). P-gp expression increased with age (P = 0.1) and white blood cell count (P = 0.1) (data not shown). Drug resistance factors and outcome of induction chemotherapy The complete remission (CR) rate of induction chemotherapy was 70% for the total study population (Table 2). Resistant disease and early death occurred in 12% and 13% of the patients, respectively, and 5% of the patients were classified as not evaluable for response because they received only one treatment cycle which did not result in CR. The CR rates were 83% for patients <60 years, 59% for patients 60 years, 76% for patients with good or intermediate karyotype, and 52% for those with poor karyotype (Table 2). White blood cell count and lactate dehydrogenase serum levels did not affect response to chemotherapy (data not shown). Expression of LRP, P-gp and bcl-2 but not MRP1 were associated with poor response to induction chemotherapy (Table 2). Patients with negative LRP had a CR rate of 79% but those with positive LRP of only 53% (P = 0.003). For patients with low, intermediate and high P-gp expression, the CR rates were 77%, 68% and 38% (P = 0.02), respectively. For patients with low, intermediate and high MRP1 expression, the CR rates were 76%, 71% and 61% (P = NS). The CR rate was 80% for patients with negative bcl-2, and 62% for those with positive bcl-2 (P = 0.03). Similar results on the association between drug resistance factors and complete remission were seen when patients with FAB M3 were excluded from the analysis (data not shown). Next, we performed logistic regression analyses on 101 patients for whom complete data were available. In the univariate analyses (Table 4), the odds ratios for complete remission were 0.3 for age 60 years (P = 0.005), 0.4 for poor karyotype (P = 0.03), 0.3 for positive LRP (P = 0.003), 0.6 for intermediate and 0.2 for high P-gp expression (P = 0.02), 0.8 for intermediate and 0.5 for high MRP1 expression (P = NS), and 0.4 for positive bcl-2 (P = 0.03). The multivariate analyses included age, karyotype and drug resistance factors (Table 4). The odds ratios for complete remission were 0.4 for age 60 years (P = 0.05), 0.3 for poor karyotype (P = 0.05), 0.3 for positive LRP (P = 0.03), 1.1 for intermediate and 0.4 for high P-gp expression (P = NS), 0.8 for intermediate and 0.5 for high MRP1 expresssion (P = NS), and 0.6 for positive bcl-2 (P = NS). Drug resistance factors and survival The associations between drug resistance factors or clinical parameters and overall survival are shown in Figure 1. Survival was better for patients <60 years than for those with age 60, and for patients with good or intermediate karyotype than for those with poor karyotype (Figure 1), but was independent of white blood cell count and lactate dehydrogenase serum levels (data not shown). Expression of LRP and of P-gp were associated with shorter overall survival. Median overall survival was 1.4 years for LRP-negative patients but only 0.7 years for LRP-positive patients (P = 0.001). Median overall survival was 1.4, 0.8 and 0.4 years for patients with low, intermediate or high P-gp expression (P = 0.01), respectively. Within the group of patients with good prognosis (patients <60 years with good or intermediate karyotype), survival was shorter in the presence than in the absence of LRP expression (median overall survival 0.9 vs 2.2 years, P = 0.02) (Figure 2). In the patients receiving consolidation chemotherapy, overall survival was longer in patients receiving high-dose cytarabine consolidation than in those receiving normal-dose cytarabine consolidation (data not shown). In patients receiving consolidation chemotherapy with high-dose cytarabine (n = 46), overall survival was shorter in LRP-positive patients than in LRP-negative patients (P = 0.1) (data not shown). Positive bcl-2 showed a trend toward shorter survival (median overall survival 0.7 vs 1.7 years, P = 0.06), whereas MRP1 expression had no impact on overall survival. In the univariate Cox regression analyses (Table 5), the relative risk for death were 2.0 for age 60 years (P = 0.008), 2.4 for poor karyotype (P = 0.006), 2.5 for positive LRP (P = 0.001), 1.8 for intermediate and 2.8 for high P-gp expression (P = 0.01), 1.9 for intermediate and 1.8 for high MRP1 expression (P = NS), and 1.7 for positive bcl-2 (P = 0.06). In the multivariate Cox regresssion analyses (Table 5), which included age, karyotype and the drug resistance factors, the relative risks for death were 2.1 for age 60 years (P = 0.01), 2.3 for poor karyotype (P = 0.02) and 2.3 for positive LRP (P = 0.01). P-gp, MRP1 and bcl-2 were not of independent prognostic significance (Table 5). In the univariate Cox regression analyses of disease-free survival, P-gp (P = 0.02) and conventional-dose cytarabine (as compared to high-dose) consolidation therapy (P = 0.006) were associated with shorter survival of the patients, and positive LRP (P = 0.08) showed a trend towards shorter survival (Table 6). No associations between age, karyotype, MRP1 or bcl-2 and disease-free survival were observed (Table 6). In the multivariate analyses, P-gp (P = 0.02) and conventional-dose cytarabine (P = 0.01) retained their prognostic significance, and LRP (P = 0.08) showed a trend toward independent prognostic value, whereas the other factors had no independent prognostic value (Table 6). Risk score Next we used the three independent predictive and prognostic factors (age 60 years, poor karyotype, LRP expression) in order to define a risk score. Dependent on the number of independent prognostic factors present in a patient, patients were divided into four groups with risk scores from 0 to 3. The outcome was different between these groups (Table 7, Figure 3). Patients with a risk score of 0, 1, 2 and 3 had complete remission rates of 93%, 75%, 47% and 33% (P < 0.001), respectively (Table 7), and a median overall survival of 2.4, 1.2, 0.6 and 0.2 years (P = 0.0001), respectively (Table 7, Figure 3). DiscussionIn the present study, age, karyotype and LRP were found to be independent predictive factors with regard to outcome of induction chemotherapy and independent prognostic factors with regard to overall survival in de novo AML. A risk score based on these three factors defined four groups of patients with different outcomes (Table 7, Figure 3). P-gp was of predictive and prognostic value in the univariate analyses but not in the multivariate analyses. Bcl-2 only predicted outcome of induction chemotherapy in the univariate analysis. MRP1 had no predictive or prognostic value. The present univariate results on LRP confirm our initial results in 82 patients,12 and are consistent with published studies.11,20,26 List et al11 reported a significant association between LRP overexpression and both an inferior response to induction chemotherapy and a trend toward shorter survival on, in comparison to our study, a more heterogeneous study population that included patients with de novo, secondary or relapsed AML. In the study by Hart et al26 LRP expression predicted response of induction chemotherapy. Borg et al20 demonstrated that LRP is an unfavorable predictive and prognostic factor in patients with de novo or secondary AML. However, our findings are in contrast to other studies including the one of the Southwest Oncology Group in younger AML patients.10,27,28 These discrepancies might be due to differences in patient populations both with regard to age and number of patients, treatment protocols, detection methods and other factors.3 Our univariate findings on the predictive and prognostic significance of P-gp are consistent with previous reports.4,5,6,7,8 The lack of an association between MRP1 expression and clinical outcome confirms our previous report on a smaller patient population, and is consistent with data from other groups.9,10,20 In a recent study by Marie's group, outcome of induction chemotherapy correlated with MRP function but not with MRP expression.28 Shorter survival in the presence of bcl-2 expression (Figure 1) has recently also been shown by others.14 The multivariate analyses revealed the independent predictive and prognostic values of age, karyotype and LRP. Whereas the predictive and prognostic values of age and karyotype are well established,29,30 our results characterize LRP as an independent predictive and prognostic factor in de novo AML. Data from multivariate analyses on LRP are currently available also from two other groups.11,20 List et al11 found that LRP but not P-gp had independent predictive significance with regard to response to induction chemotherapy. Borg et al20 performed a multivariate analysis that included age, white blood cell count, CD34, bcl-2, LRP, MRP, P-gp expression as well as rhodamine 123 efflux, FAB subtype, and cytogenetic subgroups. Age, LRP and P-gp function independently predicted for poor response to induction chemotherapy, whereas LRP, P-gp function and cytogenetics were independent prognostic factors with regard to overall survival and leukemia-free survival.20 The finding that P-gp lost its prognostic value with regard to overall survival in the present multivariate analyses is consistent with data obtained by other groups.11,30 However, we would like to stress that the lack of an independent prognostic significance of P-gp does not mean that P-gp expression is without clinical relevance in AML. It is unlikely that the lack of prognostic significance of P-gp in our study is due to the selection of the C219 antibody because we clearly demonstrated the predictive and prognostic value of P-gp in the univariate analyses. We have selected the C219 data in this study because, firstly, data from previous studies on smaller patient populations were available and, secondly, we previously did not observe significant differences between C219 and MRK16.31 Several other multivariate analyses of drug resistance factors in AML have been published.19,32,33,34,35 The parameters entered into these analyses varied but did not include LRP. We previously characterized age, MDR1 RNA expression and FAB subtype M4Eo as independent predictive factors, and age and MDR1 RNA expression as independent prognostic factors.32 These previous data are not in contrast to the present data for the following reasons: firstly, the previous multivariate analyses did not include LRP and, secondly, P-gp-positive AML was also associated with lower CR rates and shorter overall survival as well as disease-free survival as compared to P-gp-negative AML in the present study, but the differences were not statistically significant in the case of CR and overall survival. Nüssler et al33 demonstrated the prognostic significance of age, P-gp expression and t(15;17) karyotype for patients treated with protocols containing daunorubucin and vincristine, but only of age and karyotype for patients treated with intermediate-dose cytarabine/amsacrine. In our population, however, a shorter overall survival for LRP-positive patients as compared to LRP-negative patients was seen also among patients receiving high-dose cytarabine consolidation chemotherapy, but the difference did not reach the level of statistical significance, probably due to the low number of patients. Van den Heuvel-Eibrink et al19 showed that age, karyotype, P-gp and CD34 were independent predictive and prognostic factors. Hunault et al34 found that MDR1 overexpression predicted resistance to induction chemotherapy. In the Southwest Oncology Group study of elderly AML patients,30 MDR1 expression independently predicted for complete remission, but did not indicate prognosis with regard to both overall and disease-free survival. In the study by Lohri et al35 bcl-2 RNA expression and topoisomerase II RNA expression independently predicted overall survival and disease-free survival, whereas MDR1 RNA and MRP1 RNA had no impact. RNA expression independently predicted overall survival and disease-free survival, whereas MDR1 RNA and MRP1 RNA had no impact. Several explanations for the discrepancies between the various multivariate analyses exist. Firstly, the numbers and the types of factors entered into the analyses varied between the studies and probably affected the results. Secondly, patient populations differed with regard to age, disease category and sample size. In particular, the number of patients can affect the statistical power of a study. Thirdly, differences in detection levels, eg RNA vs protein vs function, and/or cut-off levels might have played a role. Finally, different treatment protocols might have affected the association between drug resistance factors and clinical outcome.33 With regard to disease-free survival, P-gp and consolidation chemotherapy with high-dose cytarabine were of independent prognostic significance. LRP showed a trend toward shorter disease-free survival. The findings on the impact of consolidation therapy are consistent with recent studies in which high-dose cytarabine consolidation chemotherapy resulted in longer disease-free36,37 and overall survival37 as compared to conventional-dose cytarabine consolidation chemotherapy. The clinical implications of our present findings are several-fold. Firstly, LRP and/or the proposed risk score might serve as prognostic factors and, in particular, might allow risk-adapted treatment in the future. For example, patients with LRP expression but otherwise good prognosis (age <60 years with good or intermediate karyotype) might be selected for more aggressive treatments, because they have worse outcome than corresponding patients without LRP expression. Secondly, the data support the multifactorial nature of drug resistance in AML. LRP was previously shown to be associated with resistance to doxorubicin, vincristine, carboplatin, cisplatin and melphalan.38 In AML cells, LRP expression correlated with intracellular daunorubucin accumulation in one study27 but not in another.39 Thus blockade of a single mechanism, such as the one of MDR1/P-gp,40,41,42 might be insufficient for improved clinical outcome. We observed several correlations between the drug resistance factors with each other (Table 3). Associations between LRP and P-gp11,12 or MRP1 expression,20,26,28 between MDR1 RNA and MRP1 RNA,26,34,35 and between P-gp and bcl-217 have also been described before. The mechanisms of these co-expressions of drug resistance factors remain to be determined. Whereas LRP was not associated with karyotype or FAB subtype, P-gp was less frequently expressed in both patients with good karyotype and certain FAB subtypes (M3, M4Eo) which is consistent with data in the literature.43,44 The optimal method for the determination of the expression of drug resistance factors in clinical samples remains a matter of debate. Immunohisto(cyto-)chemistry is a well established method in clinical oncology and also a reproducible technique for the assessment of drug resistance factors. Several studies demonstrated the reproducibility of immunocytochemistry with regard to P-gp45,46 and LRP.47 Immunocytochemical results also correlated with those obtained by flow cytometry with regard to P-gp,18,20 LRP47 and bcl-2.14 In order to resolve the issue of the optimal detection method, future comparative studies on large patient populations are required. In summary, our data indicate that LRP is a drug resistance factor with independent predictive and prognostic significance in de novo AML. LRP or the proposed risk score might be useful for the selection of patients for risk-adapted treatment in the future. Our results also support the multifactorial nature of drug resistance which will have to be considered for strategies to reverse drug resistance in this disease. Acknowledgements
This study was supported by the 'Fonds zur Förderung der wissenschaftlichen Forschung' (project number P12264-MED).
|