| Introduction Follicular lymphoma is pathologically categorized as a low-grade B cell lymphoma.1 Although clinically indolent, this lymphoma is non-curable,2,3,4 and sometimes transforms to aggressive types.5,6 Histologically, it is characterized by nodular proliferation of neoplastic B cells consisting of centroblastic and/or centrocytic like cells of the germinal centers (GC) because these tumors express various B cell-specific surface antigens of the GC B cells.7,8 In the proliferating nodules of follicular lymphoma, the presence of T cells,9 macrophages and follicular dendritic cells (FDC)7 is well documented, suggesting that these cells may contribute to a favorable environment for the growth of follicular lymphoma cells.10 Under physiological conditions, FDC form the framework for GC,11,12 and are deemed to play key roles in the maturation of antigen activated B cells.13 FDC also provide networks for the nodules of follicular lymphoma,14 which may reflect an important role in the growth of follicular lymphoma cells. FDC-like cell line HK is derived from human tonsil, and preserves the characteristics of FDC.15 Its surface phenotype is more conserved than that of ex vivo FDC, and it supports the growth and differentiation of GC B cells.16 In the presence of HK cells and with the aid of CD40L and IL-2, 4, 10, centroblasts differentiate to memory cells and plasma cells.17 Hence, we investigated whether freshly isolated follicular lymphoma cells from patients could be grown in the presence of HK cells. Here we report the establishment of a follicular lymphoma cell line, FLK-1, which is dependent on HK cells for its growth and survival, and describe its interaction with HK. Patients, materials and methodsCell lines FDC-like cell line HK is derived from follicular dendritic cells of the human tonsil.15 IMR90 is the normal human lung fibroblast cell line (American Tissue Culture Collection No. 186), SU-DHL-6, a B cell line derived from a diffuse histiocytic lymphoma which contains a BCL2 rearrangement,18 NALL-1 a pre-B cell line derived from pre-B type acute leukemia19 and MOLT4 a T cell line derived from human acute lymphoblastic leukemia.20 These cell lines were cultured with Iscove's modified Dulbecco's medium (IMDM) with 20% fetal calf serum. Cell culture on HK cells Mononuclear cells (5 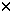 106) derived from 10 lymph nodes and one bone marrow of 11 follicular lymphoma patients were cultured on 105 HK cells in six-well plates (Costar, Cambridge, MA, USA). For control, the same number of cells from each of the sources was cultured without HK cells in six-well plates. 106) derived from 10 lymph nodes and one bone marrow of 11 follicular lymphoma patients were cultured on 105 HK cells in six-well plates (Costar, Cambridge, MA, USA). For control, the same number of cells from each of the sources was cultured without HK cells in six-well plates. Clinical course of the case from which FLK-1 derived A 47-year-old Japanese female, hospitalized in November 1993, because of systemic lymphadenopathy was diagnosed as having follicular mixed small and large cell type lymphoma (Figure 1). She underwent CHOP therapy and attained complete remission. In June 1996, cervical lymphadenopathy was noted but the administration of oral etoposide once again induced complete remission. In November 1997, the patient showed signs of a second recurrence in the anterior chest mass, bone and bone marrow. She again underwent therapy, but the bone lesion became refractory from the end of 1997, and in spite of intensive chemotherapy, she died of the disease in September 1998. Post-mortem bone marrow aspiration demonstrated extensive infiltration of lymphoma cells (Figure 2a). These lymphoma cells were used to establish FLK-1 after informed consent had been obtained from the patient's family. Light microscopy and cell morphology Tissue samples were fixed in 10% formalin and embedded in paraffin. Sections (5 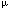 m thick) were stained with hematoxylin and eosin (H&E). A total of 104 cells was cytocentrifuged on to slides for the analysis of FLK-1. May–Grünwald-Giemsa staining was used for the morphological analysis. m thick) were stained with hematoxylin and eosin (H&E). A total of 104 cells was cytocentrifuged on to slides for the analysis of FLK-1. May–Grünwald-Giemsa staining was used for the morphological analysis. Detection of mycoplasma and Epstein–Barr virus (EBV) in FLK-1 The cells were examined for mycoplasma contamination with the DNA fluorochrome staining method (ICN Hoechst Stain Kit; ICN Biomedicals, Aurora, OH, USA), and for EBV infection by means of detection of EBV-encoded small RNAs (EBER) in formalin-fixed cytocentrifugation specimens using in situ hybridization of (EBER) oligonucleotides. Hybridization was performed with a cocktail of fluorescein isothiocyanate (FITC)-labeled EBER oligonucleotides (one oligonucleotide corresponding to EBER1 and one to EBER2, both 30 bases long) (Dako A/S code Y 017, Dako, Santa Fe, CA, USA). Hybridization products were detected by using a mouse monoclonal anti-FITC (Dako M878) and a Vectastain ABC Kit (Vector, Burlingame, CA, USA). Antibodies Anti-CD40 (G28.5) was generously provided by Dr JA Ledbetter (Bristol-Myers Squibb Pharmaceutical Research Institute, Seattle, WA, USA). Anti-CD44 (NKI-P2) was a generous gift from Dr CG Figdor (The Netherlands Cancer Institute, Amsterdam, The Netherlands). Anti-ICAM-1 (RR1/1) and anti-LFA-1 (R3.1) were kind gifts from Dr R Rothlein (Boehringer-Ingelheim, Ridgefield, CT, USA). FITC- or PE-conjugated anti-CD2 (S5.2), anti-CD3 (SK7), anti-CD4 (SK3), anti-CD5 (L17F12), anti-CD8 (SK1), anti-CD10 (W8E7), anti-CD14 (MP9), anti-CD19 (4G7), anti-CD20 (L27), anti-CD23 (EBVCS-5), anti-CD25 (2A3), anti-CD33 (P.67.6) and anti-HLA-DR (L243) were purchased from Becton Dickinson (San Jose, CA, USA). FITC- or PE-conjugated anti-CD38 (HIT2) and anti-CD95 (DX2) were purchased from Pharmingen (San Diego, CA, USA). FITC-conjugated anti-Ig, anti-Ig and biotinylated PNA were purchased from Dako (Carpinteria, CA, USA), and FITC-conjugated goat anti-human IgA, IgD, IgG, and IgM from Southern Biotechnology Associates (Birmingham, AL, USA). Anti-BCL2 was purchased from Boehringer Mannheim (Indianapolis, IN, USA), and isotype controls were purchased as follows: unconjugated or FITC-conjugated isotype controls from Pharmingen and PE-or biotin conjugated isotype controls and FITC-conjugated isotype control for anti-Ig and anti-Ig from Dako. Flowcytometry Flow cytometric analysis was performed on a FACScan (Becton Dickinson) with CELLQuest software. The cytometer was calibrated with computer software FACSComp using CaliBRITE beads (Becton Dickinson). Cytogenetics Chromosomal analysis of the original lymphoma was performed at the Special Reference Laboratory (Tokyo, Japan). FLK-1 was analyzed with conventional methods and karyotyped by using the International System for Cytogenetic Nomenclature (ISCN).21 DNA extraction and Southern blot analysis For the extraction of high molecular weight DNA, the original bone marrow tumor and FLK-1 cells were treated with proteinase K and extracted with phenol and chloroform. Purified DNA was digested with BamHI, EcoRI, or HindIII (Boehringer Mannheim, Mannheim, Germany), electrophoresed on a 0.8% agarose gel and transferred to nylon membranes (Hybond-N+, Amersham-Japan, Tokyo, Japan). Probes for hybridization consisted of the MBR for the human BCL2 gene and JH for the human Ig heavy chain (IgH) gene as previously described.22 Cell growth curve 104 feeder cells per well were cultured in a 24-well cluster dish (Costar, Cambridge, MA, USA) on day -1. On day 0, after removal of the medium, 1 ml of cell suspension at a concentration of 104/ml was plated to each well in triplicate. Floating viable cells were counted on days 3, 5, 7 and 9. For the HK cell dependent growth of FLK-1, 2 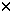 104 FLK-1 cells were cultured in triplicate cultures in a 24-well cluster dish (Corning, Corning, NY, USA) with different numbers of HK cells in RPMI 1640 (Irvine Scientific, Santa Ana, CA, USA) supplemented with 10% fetal calf serum (Life Technologies, Grand Island, NY, USA), 2 mM glutamine, 100 U/ml penicillin G, and 100 104 FLK-1 cells were cultured in triplicate cultures in a 24-well cluster dish (Corning, Corning, NY, USA) with different numbers of HK cells in RPMI 1640 (Irvine Scientific, Santa Ana, CA, USA) supplemented with 10% fetal calf serum (Life Technologies, Grand Island, NY, USA), 2 mM glutamine, 100 U/ml penicillin G, and 100 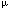 g/ml streptomycin (Irvine Scientific). The viable cells were counted on days 3 and 4. g/ml streptomycin (Irvine Scientific). The viable cells were counted on days 3 and 4. Detection of apoptotic cells FLK-1 cells (1 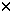 105) were cultured in the presence or absence of HK cells (2 105) were cultured in the presence or absence of HK cells (2 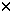 105) for 2 days, and then washed and stained with FITC-labeled Annexin V (AN) and propidium iodide (PI) (Trevigen, Gaithersburg, MD, USA). The extent of apoptosis was measured by means of flowcytometric analysis using FACScan (Becton Dickinson). Populations were selected on the basis of the fact that cells expressing phosphatidylserine on the outer leaflet of cell membranes bind AN, and cells with a compromised cell membrane allow PI to bind to the cellular DNA. Thus, the three populations of AN-/PI-, AN+/PI+, and AN+/PI+ have been found to correspond to, respectively, live cells, early apoptotic cells, and both late apoptotic cells and necrotic cells.23,24 105) for 2 days, and then washed and stained with FITC-labeled Annexin V (AN) and propidium iodide (PI) (Trevigen, Gaithersburg, MD, USA). The extent of apoptosis was measured by means of flowcytometric analysis using FACScan (Becton Dickinson). Populations were selected on the basis of the fact that cells expressing phosphatidylserine on the outer leaflet of cell membranes bind AN, and cells with a compromised cell membrane allow PI to bind to the cellular DNA. Thus, the three populations of AN-/PI-, AN+/PI+, and AN+/PI+ have been found to correspond to, respectively, live cells, early apoptotic cells, and both late apoptotic cells and necrotic cells.23,24 For detection of the DNA ladder, 106 FLK-1 cells were cultured without HK cells for 3 days, and DNA was purified from the collected cells as described elsewhere.25 Briefly, the cells were washed with phosphate buffered saline and lysed with 500 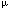 l of 5 mM Tris pH 7.4, 20 mM EDTA and 0.5% Triton X-100. After phenol and chloroform extraction, the aqueous phase was precipitated with ethanol, dissolved and treated with 20 l of 5 mM Tris pH 7.4, 20 mM EDTA and 0.5% Triton X-100. After phenol and chloroform extraction, the aqueous phase was precipitated with ethanol, dissolved and treated with 20 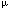 g/ml of RNaseA. Half of the total volume was electrophoresed on a 1.5% agarose gel. g/ml of RNaseA. Half of the total volume was electrophoresed on a 1.5% agarose gel. Transwell culture HK cells (105) were seeded into a six-well cluster dish (Transwell, Costar) on day -1. On day 0, the medium was changed to 2.6 ml of fresh medium. Next, the bottom cup with a 0.4 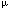 m microporous membrane was put in place followed by the inoculation of 1.5 ml of 5 m microporous membrane was put in place followed by the inoculation of 1.5 ml of 5 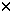 104 FLK-1 cell suspension. The positive control consisted of the culture without the inner cup. The number of viable cells was counted every 2 days by means of the trypan blue dye exclusion test, and when the number of cells exceeded 106/well, one-third of the cell suspension was transferred to a new dish. The number of cells was counted four times, and the average of three independent wells 104 FLK-1 cell suspension. The positive control consisted of the culture without the inner cup. The number of viable cells was counted every 2 days by means of the trypan blue dye exclusion test, and when the number of cells exceeded 106/well, one-third of the cell suspension was transferred to a new dish. The number of cells was counted four times, and the average of three independent wells 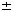 standard error was plotted. standard error was plotted. Soluble factor(s) supporting FLK-1 cell growth The supernatant was harvested from 6.25 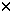 105 HK cells cultured with 4.5 ml culture medium for 24 h in a 10-cm Petri dish, and then filtered, diluted with fresh media and cryopreserved at -70°C. It was thawed just before use. In order to make the culture system consistent, Transwell culture was also used for the assay. The bottom cup with a 0.4 105 HK cells cultured with 4.5 ml culture medium for 24 h in a 10-cm Petri dish, and then filtered, diluted with fresh media and cryopreserved at -70°C. It was thawed just before use. In order to make the culture system consistent, Transwell culture was also used for the assay. The bottom cup with a 0.4 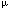 m microporous membrane was put into 1.5 ml medium in a 12-well cluster dish, and 0.5 ml of the supernatant containing 5 m microporous membrane was put into 1.5 ml medium in a 12-well cluster dish, and 0.5 ml of the supernatant containing 5 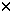 104 FLK-1 cells was added to the cup. The final concentrations of the supernatant were 20%, 40% and 80%. The medium in the outer chamber was replenished every day with the cryopreserved supernatant. For control, FLK-1 cells were also cultured in fresh medium with or without HK cells. On day 3, 100 104 FLK-1 cells was added to the cup. The final concentrations of the supernatant were 20%, 40% and 80%. The medium in the outer chamber was replenished every day with the cryopreserved supernatant. For control, FLK-1 cells were also cultured in fresh medium with or without HK cells. On day 3, 100 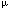 l of the cell suspension in the inner cups was transferred to 96-well plates. The number of viable cells was measured with a colorimetric assay (CellTiter 96; Aqueous Non-Radioactive Cell Proliferation Assay Kit, Promega, Madison, WI, USA). The mean values l of the cell suspension in the inner cups was transferred to 96-well plates. The number of viable cells was measured with a colorimetric assay (CellTiter 96; Aqueous Non-Radioactive Cell Proliferation Assay Kit, Promega, Madison, WI, USA). The mean values 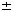 standard error of the absorbance at 490 nm in the quadruplicate wells were plotted. standard error of the absorbance at 490 nm in the quadruplicate wells were plotted. Cytokine assay On day 0, 25 ml of 105/ml FLK-1 cell suspension was seeded into a 10-cm Petri dish which had been inoculated with 6.25 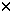 105 HK cells on day -1. FLK-1 cell cultures without HK cells or with only the medium containing HK cells were also performed. On days 1, 3 and 5, 3 ml of each culture was harvested, centrifuged at 3000 r.p.m. for 10 min, and cryopreserved until the cytokine enzyme immunosorbent assay. The following assay kits were used for this study: for IL-1 105 HK cells on day -1. FLK-1 cell cultures without HK cells or with only the medium containing HK cells were also performed. On days 1, 3 and 5, 3 ml of each culture was harvested, centrifuged at 3000 r.p.m. for 10 min, and cryopreserved until the cytokine enzyme immunosorbent assay. The following assay kits were used for this study: for IL-1 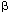 , Japan Immunoresearch Laboratories (Takasaki, Japan); for IL-1ra, R&D Systems (Minneapolis, MN, USA); and for IL-1 , Japan Immunoresearch Laboratories (Takasaki, Japan); for IL-1ra, R&D Systems (Minneapolis, MN, USA); and for IL-1  , IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IL-15, interferon- , IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IL-15, interferon- , interferon- , interferon-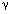 , TNF- , TNF- , G-CSF, and GM-CSF, Otsuka Assay Laboratories (Tokushima, Japan). , G-CSF, and GM-CSF, Otsuka Assay Laboratories (Tokushima, Japan). ResultsEstablishment of FLK-1 cell line Cells from half of the lymph nodes proliferated transiently on HK cells, but those without HK did not show any significant proliferation, while the cells from the remaining lymph nodes did not proliferate, even with HK cells. All of the cells had ceased to grow by 1 month from the start of the culture. However, cells from bone marrow invasion proliferated vigorously without clump formation and most of them were floating, but the lymphoma cells without HK did not grow. Proliferating cells were split every 3 to 4 days at 1:3 to 1:4 and kept growing for more than 6 months in the presence of HK cells. These cells could be diluted to a concentration of only 104/ml, and continued to grow up to a confluent cell density of 2 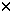 106/ml. Growing cells were frozen in the presence of 4% human albumin, 5% DMSO and 6% hydroxyethyl starch (CP-1; Kyokuto Pharmaceutical, Tokyo, Japan) for preservation. The frozen cells were thawed and examined for their dependency on HK cells. The same levels of dependency were found to be maintained for more than 6 months, as was the identical growth pattern. The cells were not contaminated with mycoplasma and found to be free of EBV. This cell line was designated FLK-1, and will be available upon request after written permission to use HK cells has been obtained from the Alton Ochsner Medical Foundation (Dr YS Choi) because HK cells are patented. 106/ml. Growing cells were frozen in the presence of 4% human albumin, 5% DMSO and 6% hydroxyethyl starch (CP-1; Kyokuto Pharmaceutical, Tokyo, Japan) for preservation. The frozen cells were thawed and examined for their dependency on HK cells. The same levels of dependency were found to be maintained for more than 6 months, as was the identical growth pattern. The cells were not contaminated with mycoplasma and found to be free of EBV. This cell line was designated FLK-1, and will be available upon request after written permission to use HK cells has been obtained from the Alton Ochsner Medical Foundation (Dr YS Choi) because HK cells are patented. Morphological and phenotypical analysis The histology of lymphoma cells in the bone marrow showed a focal invasive pattern of blastic cells with conspicuous nucleoli, while FLK-1 cells featured basophilic cytoplasm with cleaved nuclei (Figure 2b). The phenotypical analysis of the original lymphoma and FLK-1 demonstrated the expression of CD19, CD20, sIgG, CD38 and HLA-DR type which is characteristic of centroblastic cells (Figure 3). Chromosomal and genotypical analysis FLK-1 demonstrated complicated chromosomal abnormalities (Figure 4a), including t(14;18)(q32;q21) and del(13)(q12q14), which were also seen in the original lymphoma cells in the bone marrow (data not shown). The BCL2 gene rearrangement was identified by means of Southern blot analysis using the BCL2 MBR probe, and the presence of two identical rearranged bands was demonstrated in both the original lymphoma and FLK-1 (Figure 4b). The rearrangement pattern of the immunoglobulin heavy chain (IgH) gene was identical, while one of the rearranged bands of the BCL2 gene showed comigration with the rearranged band of IgH (arrows). These findings indicate that FLK-1 was derived from the lymphoma cells. HK-dependent growth HK cell dependency of FLK-1 was found to be dose-dependent and findings on day 4 are shown (Figure 5). Next, to investigate whether the dependency of FLK-1 was unique to HK, and whether the supporting activity of HK was confined to FLK-1, the growth pattern of various hematopoietic cell lines on HK or those on human fibroblast cell line IMR90 was examined. Figure 6a shows that FLK-1 proliferated vigorously on HK, while it ceased to grow on IMR90 or in the absence of feeder cells. In addition, a human skin fibroblast cell line, SK25, also failed to support growth of FLK-1 (data not shown). PreB-ALL cell line NALL1 (Figure 6b) and T-ALL cell line MOLT4 (Figure 6c) showed similar growth curves on HK, IMR90 or without any feeder cells. SUDHL6 (Figure 6d), derived from a diffuse histocytic lymphoma with BCL2 translocation, also showed no growth dependency on HK or IMR90. These findings indicate that the growth promoting property of HK cells is specific to FLK-1. Induction of apoptosis by deprivation of HK cells When HK cells were deleted from the culture, FLK-1 cells stopped growing and some of the cells began to die. The cell death mechanism was examined with the Annexin V/PI staining method (Figure 7a). The cultured cells were stained with FITC-labeled Annexin V and propidium iodide (PI) and analyzed on a FACS scan. During a 48-h absence of HK cells, Annexin V-PI- viable cells decreased from 83% to 53% with a concurrent increase in Annexin V+PI- (early apoptotic) cells from 7% to 34%. Electrophoresis of DNA extracted on day 3 without HK showed a DNA ladder (Figure 7b). These data indicate that the deprivation of HK induced apoptosis of FLK-1. Soluble factor(s) sustain the growth of FLK-1 We also examined whether direct contact is required for growth dependency of FLK-1 on HK cells. By culturing FLK-1 in inner cups of Transwell dish which prevented direct cell-to-cell contact with HK cells, the capability of the HK cells to support FLK-1 growth was examined (Figure 8). FLK-1 proliferated at the same rate as in the culture irrespective of whether it was physically separated from HK or not until day 5. However, after day 5, FLK-1 without direct contact started to show cell death and growth retardation. Finally all of the cells had died within 3 weeks. FLK-1 without HK cells or conditioned medium showed growth retardation from the early phase, ceased to grow after day 9, and all of the cells had died by day 17. To confirm that the growth promotion was derived from soluble factor(s) produced by HK, FLK-1 was also cultured together with the conditioned medium of HK within the cups of the Transwell dish. Various proportions of the conditioned medium supported the growth of FLK-1 in a dose-dependent manner (Figure 9). With the supernatant accounting for 80% of the conditioned medium, the number of cells increased to the same level as that with the culture in the presence of HK. These findings indicate that the growth of FLK-1 is dependent of HK cells, and that this growth is partially sustained by soluble factor(s) in the short term even without direct contact. Cytokine detection in the culture media Although HK cells are necessary for long-term growth of FLK-1, supernatant of HK supported the growth transiently, suggesting that the cell-to-cell interaction is necessary for long-term growth. In the culture of FLK-1, a considerable portion of FLK-1 proliferate in floating state. The concentrations of various cytokines in the culture of FLK-1, HK or FLK-1 with HK were examined (Table 1). IL-6, IL-8 and IL-15 were secreted by HK cells, but other cytokines which were also reported to activate the growth of B cells were not detected. On the contrary, IL-10 was detected only in the supernatant of co-culture, suggesting that cell-to-cell interaction triggered secretion of cytokine. DiscussionFollicular lymphoma cells are present in the network of FDC within the follicles. Even in the bone marrow lesion of low-grade B cell lymphoma, FDC clusters are found,26 suggesting the importance of FDC for the growth of lymphoma cells. Our culture of lymphoma cells with FDC cell line HK showed that, indeed, cells from half of the lymph nodes proliferated transiently on HK cells, but those without HK did not, indicating that FDC can, at least in part, support lymphoma cell growth. Since all of the cells from the lymph nodes had ceased to grow by 1 month after the start of the culture, it is obvious that HK cells are not enough to maintain their growth. Interestingly, lymphoma cells from bone marrow invasion of a patient proliferated vigorously in floating state with HK cells, suggesting that these particular lymphoma cells may have undergone some genetic change for their growth on HK cells. Our analysis of the follicular lymphoma cell line FLK-1 derived from the lymphoma cells of bone marrow invasion indicated that this cell line was dependent for its growth upon the follicular dendritic cell line HK. Furthermore, the growth promoting activity of HK was limited to FLK-1 among all the cell lines tested, including B or T cell leukemia and diffuse lymphoma cell lines. When HK was removed from the culture, the growth rate of FLK-1 was significantly reduced and most of the cells died within 2 weeks. The morphology of the dying cells indicated that apoptosis plays at least a partial role. Although BCL2 protein was shown to be expressed in FLK-1, BCL2 by itself does not appear to be sufficient for inhibiting apoptosis of this lymphoma cell line. The Transwell experiments revealed that the growth of FLK-1 was maintained for a short period by soluble factor(s) produced by HK. It has been shown that the supernatant of HK augments the proliferation of B cells treated with anti-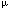 or anti-CD40.16 Since HK produces IL-6, we examined whether IL-6 can replace the growth promoting activity of HK, but IL-6 alone was found not to support FLK-1 growth (data not shown), suggesting that some other as yet unknown factor(s) mediate(s) lymphoma cell growth. In the Transwell experiment, where micropore membranes prevented FLK-1 from direct contact with HK, FLK-1 did not survive for a long time. This indicates that direct cell-to-cell contact is necessary for long-term growth. Under physiological conditions, FDC can stimulate the survival and proliferation of normal B cells by cell-to-cell interaction.27,28,29 Hence, it is conceivable that both soluble factor(s) and cell-to-cell interaction may be necessary for follicular lymphoma cells to grow. Indeed, co-cultivation of HK and FLK-1 produced IL-10, which was not produced by HK or FLK-1 separately, indicating that cell-to-cell interaction may trigger factor(s) to help cells survive. or anti-CD40.16 Since HK produces IL-6, we examined whether IL-6 can replace the growth promoting activity of HK, but IL-6 alone was found not to support FLK-1 growth (data not shown), suggesting that some other as yet unknown factor(s) mediate(s) lymphoma cell growth. In the Transwell experiment, where micropore membranes prevented FLK-1 from direct contact with HK, FLK-1 did not survive for a long time. This indicates that direct cell-to-cell contact is necessary for long-term growth. Under physiological conditions, FDC can stimulate the survival and proliferation of normal B cells by cell-to-cell interaction.27,28,29 Hence, it is conceivable that both soluble factor(s) and cell-to-cell interaction may be necessary for follicular lymphoma cells to grow. Indeed, co-cultivation of HK and FLK-1 produced IL-10, which was not produced by HK or FLK-1 separately, indicating that cell-to-cell interaction may trigger factor(s) to help cells survive. Several B-lymphoma cell lines have been established so far from follicular lymphoma,30,31,32,33,34 but they do not require FDC for their growth. The FLK-1 cell line is thus the first follicular lymphoma cell line with dependency on FDC line HK. The unique property of HK cell dependency of FLK-1 cells should help the exploration of the important mechanism of lymphoma–FDC interaction, which has been ignored in conventional cultures without FDC. Therefore, the FLK-1 cell line established in the present study should serve as an effective system for the identification of soluble factor(s), receptor(s), and cell surface molecules that mediate interaction between FLK-1 and HK. It is also important to examine if other dendritic cells serve like HK cells.35 Such studies should provide further insights into the molecular pathogenesis of follicular lymphoma, and thus lead to new and improved therapeutic strategies. Acknowledgements
The authors thank M Ando, T Kobayashi and H Ishida for their technical assistance. This research was supported in part by a Grant-in-aid from the Ministry of Health and Welfare and a Grant-in-aid from the Ministry of Education, Science and Culture of Japan.
|