|
K Schmiegelow & U Bretton-Meyer
The Laboratory for Pediatric Oncology, The Pediatric Clinic II, The Juliane Marie Centre, The National University Hospital, Rigshospitalet, Copenhagen, Denmark
Correspondence to: K Schmiegelow, The Pediatric Clinic II, JMC-4064, The National University Hospital, Rigshospitalet, Blegdamsvej 9, DK-2100 Copenhagen, Denmark; Fax: +45 3545 4673
|
Through inhibition of purine de novo synthesis and enhancement of 6-mercaptopurine (6MP) bioavailability high-dose methotrexate (HDM) may increase the incorporation into DNA of 6-thioguanine nucleotides (6TGN), the cytoxic metabolites of 6MP. Thus, coadministration of 6MP could increase myelotoxicity following HDM. Twenty-one children with standard risk (SR) and 25 with intermediate risk (IR) acute lymphoblastic leukemia (ALL) were studied. During consolidation therapy they received either three courses of HDM at 2 week intervals without concurrent oral 6MP (SR-ALL) or four courses of HDM given at 2 week intervals with 25 mg/m2 of oral 6MP daily (IR-ALL). During the first year of maintenance with oral 6MP (75 mg/m2/day) and oral MTX (20 mg/m2/week) they all received five courses of HDM at 8 week intervals. In all cases, HDM consisted of 5000 mg of MTX/m2 given over 24 h with intraspinal MTX and leucovorin rescue. Erythrocyte levels of 6TGN (E-6TGN) and methotrexate (E-MTX) were, on average, measured every second week during maintenance therapy. When SR consolidation (6MP: 0 mg), IR consolidation (6MP: 25 mg/m2), and SR/IR maintenance therapy (6MP: 75 mg/m2) were compared, white cell and absolute neutrophil count (ANC) nadir, lymphocyte count nadir, thrombocyte count nadir, and hemoglobin nadir after HDM decreased significantly with increasing doses of oral 6MP. Three percent of the HDM courses given without oral 6MP (SR consolidation) were followed by an ANC nadir <0.5 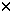 109/l compared to 50% of the HDM courses given during SR/IR maintenance therapy. Similarly, only 13% of the HDM courses given as SR-ALL consolidation induced a thrombocyte count nadir <100 109/l compared to 50% of the HDM courses given during SR/IR maintenance therapy. Similarly, only 13% of the HDM courses given as SR-ALL consolidation induced a thrombocyte count nadir <100 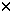 109/l compared to 58% of the HDM courses given during maintenance therapy. The best-fit model to predict the ANC nadir following HDM during maintenance therapy included the dose of 6MP prior to HDM ( 109/l compared to 58% of the HDM courses given during maintenance therapy. The best-fit model to predict the ANC nadir following HDM during maintenance therapy included the dose of 6MP prior to HDM (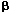 = -0.017, P = 0.001), the average ANC level during maintenance therapy ( = -0.017, P = 0.001), the average ANC level during maintenance therapy (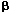 = 0.82, P = 0.004), and E-6TGN ( = 0.82, P = 0.004), and E-6TGN (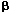 = -0.0029, P = 0.02). The best-fit model to predict the thrombocyte nadir following HDM during maintenance therapy included only mPLATE ( = -0.0029, P = 0.02). The best-fit model to predict the thrombocyte nadir following HDM during maintenance therapy included only mPLATE (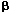 = 0.0057, P = 0.046). In conclusion, the study indicates that reductions of the dose of concurrently given oral 6MP could be one way of reducing the risk of significant myelotoxicity following HDM during maintenance therapy of childhood ALL. Leukemia (2001) 15, 74–79. = 0.0057, P = 0.046). In conclusion, the study indicates that reductions of the dose of concurrently given oral 6MP could be one way of reducing the risk of significant myelotoxicity following HDM during maintenance therapy of childhood ALL. Leukemia (2001) 15, 74–79.
|